Home > Press > Metal oxide ‘can transform’
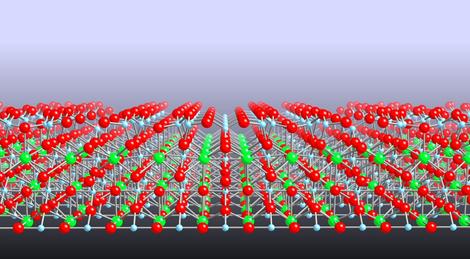 |
| An atomic model of the surface of strontium titanate |
Abstract:
An international team, including Oxford University scientists, has been investigating what happens to the top layer of atoms on the surface of a material.
Metal oxide ‘can transform’
UK | Posted on February 15th, 2010The material is strontium titanate: a complex metal oxide that many researchers are interested in because of its ability to split water into hydrogen and oxygen with sunlight and its potential for use in electronic devices.
The team used a variety of techniques including scanning tunneling microscopy (STM) to directly ‘see' the arrangement of surface atoms. Their observations, reported in this week's Nature Materials, reveal a series of structures with a surprisingly close and orderly arrangement.
‘In most materials, when you create a surface, the top layer of atoms rearrange to different positions from those in the rest of the material. This rearrangement of atoms is usually locked into a particular configuration that will minimise the surface energy.' said Dr Martin Castell of Oxford University's Department of Materials, an author of the paper. ‘However, this is not the case for the surface of strontium titanate that we have been studying. This surface forms a whole family of different structures. Chemists would call these structures a homologous series - something that is routinely observed in the bulk of crystals, but not until now on the surface.'
These ‘transformations' could prove very important to researchers hoping to use strontium titanate in order to build new kinds of nanoelectronic devices or to grow thin films.
The report also suggests that the techniques developed by the researchers could make it possible to predict the surface structures of other oxides.
'We have needed to use many different sophisticated experimental and theoretical approaches to solve this problem,' said Dr Castell. 'Our aim is to continue to work closely with our collaborators at Northwestern University in the US to solve related materials problems.'
The research was conducted by a team was led by Dr Martin Castell of Oxford University UK and Professor Laurence Marks and Professor Ken Poeppelmeier of Northwestern University, USA.
A report of the research, ‘A homologous series of structures on the surface of SrTiO3 (110)', is published in this week's Nature Materials.
####
About Oxford University
Welcome to the University of Oxford. People from all walks of life and all parts of the world have been visiting us for nine centuries and we are delighted that via this website you are joining that long tradition. Oxford was the first University in the English-speaking world. Our aim is to remain at the forefront of centres of learning, teaching and research.
Oxford’s remarkable global appeal continues to grow. Students from more than a hundred and forty countries and territories make up a student population of over twenty thousand. Over a third comes from outside the United Kingdom.
For more information, please click here
Copyright © Oxford University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Thin films
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanoelectronics
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() Interdisciplinary: Rice team tackles the future of semiconductors Multiferroics could be the key to ultralow-energy computing October 6th, 2023
Interdisciplinary: Rice team tackles the future of semiconductors Multiferroics could be the key to ultralow-energy computing October 6th, 2023
![]() Key element for a scalable quantum computer: Physicists from Forschungszentrum Jülich and RWTH Aachen University demonstrate electron transport on a quantum chip September 23rd, 2022
Key element for a scalable quantum computer: Physicists from Forschungszentrum Jülich and RWTH Aachen University demonstrate electron transport on a quantum chip September 23rd, 2022
![]() Reduced power consumption in semiconductor devices September 23rd, 2022
Reduced power consumption in semiconductor devices September 23rd, 2022
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Alliances/Trade associations/Partnerships/Distributorships
![]() Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
![]() University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








