Home > Press > New designs for solid-state electrolytes may soon revolutionize the battery industry: Scientists achieve monumental improvements in lithium-metal-chloride solid-state electrolytes
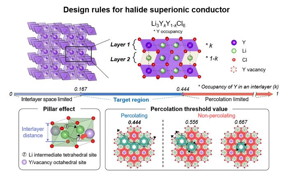 |
| The arrangement of metal ions (yttrium in this case) within each layer affects the ionic conductivity. To ensure the unobstructed movement of lithium ions, the number of metal ions occupying available sites within each layer should be less than 0.444. Furthermore, to create a sufficiently wide pathway for lithium ions within each layer, the occupancy of metal ions should be more than 0.167. Therefore, achieving an occupancy of metal ions between 0.167 and 0.444 within each layer results in a conductive layer with high ionic conductivity. CREDIT Institute for Basic Science |
Abstract:
Researchers led by Professor KANG Kisuk of the Center for Nanoparticle Research within the Institute for Basic Science (IBS), have announced a major breakthrough in the field of next-generation solid-state batteries. It is believed that their new findings will enable the creation of batteries based on a novel chloride-based solid electrolyte that exhibits exceptional ionic conductivity.
New designs for solid-state electrolytes may soon revolutionize the battery industry: Scientists achieve monumental improvements in lithium-metal-chloride solid-state electrolytes
Daejeon, Republic of Korea | Posted on November 3rd, 2023A pressing concern with current commercial batteries their reliance on liquid electrolytes, which leads to flammability and explosion risks. Therefore, the development of non-combustible solid electrolytes is of paramount importance for advancing solid-state battery technology. As the world gears up to regulate internal combustion engine vehicles and expand the use of electric vehicles in the ongoing global shift toward sustainable transportation, research into the core components of secondary batteries, particularly solid-state batteries, has gained significant momentum.
To make solid-state batteries practical for everyday use, it is crucial to develop materials with high ionic conductivity, robust chemical and electrochemical stability, and mechanical flexibility. While previous research successfully led to sulfide and oxide-based solid electrolytes with high ionic conductivity, none of these materials fully met all these essential requirements.
In the past, scientists have also explored chloride-based solid electrolytes, known for their superior ionic conductivity, mechanical flexibility, and stability at high voltages. These properties led some to speculate that chloride-based batteries are the most likely candidates for solid-state batteries. However, these hopes quickly died out, as the chloride batteries were considered impractical due to their heavy reliance on expensive rare earth metals, including yttrium, scandium, and lanthanide elements, as secondary components.
To address these concerns, the IBS research team looked at the distribution of metal ions in chloride electrolytes. They believed the reason trigonal chloride electrolytes can achieve low ionic conductivity is based on the variation of metal ion arrangements within the structure.
They first tested this theory on lithium yttrium chloride, a common lithium metal chloride compound. When the metal ions were positioned near the pathway of lithium ions, electrostatic forces caused obstruction in their movement. Conversely, if the metal ion occupancy was too low, the path for lithium ions became too narrow, impeding their mobility.
Building on these insights, the research team introduced strategies to design electrolytes in a way that mitigates these conflicting factors, ultimately leading to the successful development of a solid electrolyte with high ionic conductivity. The group went further to successfully demonstrate this strategy by creating a lithium-metal-chloride solid-state battery based on zirconium, which is far cheaper than the variants that employ rare earth metals. This was the first instance where the significance of the metal ions arrangement on a material’s ionic conductivity was demonstrated.
This research brings to light the often-overlooked role of metal ion distribution in the ionic conductivity of chloride-based solid electrolytes. It is expected that the IBS Center’s research will pave the way for the development of various chloride-based solid electrolytes and further drive the commercialization of solid-state batteries, promising improved affordability and safety in energy storage.
Corresponding author KANG Kisuk states, “This newly discovered chloride-based solid electrolyte is poised to transcend the limitations of conventional sulfide and oxide-based solid electrolytes, bringing us one step closer to the widespread adoption of solid-state batteries.”
This research was published on November 3, 2023, in Science, which is one of the world's most prestigious scientific journals.
####
For more information, please click here
Contacts:
William Suh
Institute for Basic Science
Office: 82-010-379-37830
Copyright © Institute for Basic Science
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Possible Futures
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Discoveries
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Automotive/Transportation
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








