Home > Press > Previously unknown pathway to batteries with high energy, low cost and long life: Newly discovered reaction mechanism overcomes rapid performance decline in lithium-sulfur batteries
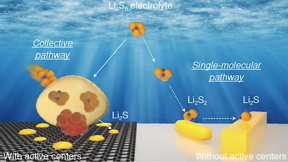 |
| Different reaction pathways from lithium polysulfide (Li₂S₆) to lithium sulfide (Li₂S) in lithium-sulfur batteries with (left) and without (right) catalyst in sulfur cathode. CREDIT (Image by Argonne National Laboratory.) |
Abstract:
Scientists discover surprising pathway to better lithium-sulfur batteries by visualizing reactions at the atomic scale.
Previously unknown pathway to batteries with high energy, low cost and long life: Newly discovered reaction mechanism overcomes rapid performance decline in lithium-sulfur batteries
Lemont, IL | Posted on September 8th, 2023The road from breakthrough in the lab to practical technology can be a long and bumpy one. The lithium-sulfur battery is an example. It has notable advantages over current lithium-ion batteries powering vehicles. But it has yet to dent the market despite intense development over many years.
That situation could change in the future thanks to the efforts of scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory. Over the past decade, they have made several pivotal discoveries related to lithium-sulfur batteries. Their latest revelation, published in Nature, unlocks a previously unknown reaction mechanism that addresses a major shortcoming — the batteries’ very short lifetimes.
Gui-Liang Xu, chemist in Argonne’s Chemical Sciences and Engineering division, stated that “Our team’s efforts could bring the U.S. one large step closer to a greener and more sustainable transportation landscape.”
Lithium-sulfur batteries offer three significant advantages over current lithium-ion batteries. Firstly, they can store two to three times more energy in a given volume, resulting in longer vehicle ranges. Secondly, their lower cost, facilitated by the abundance and affordability of sulfur, makes them economically viable. Lastly, these batteries do not rely on critical resources like cobalt and nickel, which may face shortages in the future.
Despite these benefits, transitioning from laboratory success to commercial viability has proven elusive. Laboratory cells have shown promising results, but when scaled up to commercial size, their performance rapidly declines with repeated charge and discharge.
The underlying cause of this performance decline lies in the dissolution of sulfur from the cathode during discharge, leading to the formation of soluble lithium polysulfides (Li2S6). These compounds flow into the lithium metal negative electrode (anode) during charging, further exacerbating the issue. Consequently, the loss of sulfur from the cathode and alterations in the anode composition significantly hinder the battery’s performance during cycling.
In a recent earlier study, Argonne scientists developed a catalytic material that, when added in a small amount to the sulfur cathode, essentially eliminated the sulfur loss problem. While this catalyst showed promise in both laboratory and commercial-size cells, its atomic-scale working mechanism remained an enigma until now.
The team’s most recent research shed light on this mechanism. In the absence of the catalyst, lithium polysulfides form at the cathode surface and undergo a series of reactions, ultimately converting the cathode to lithium sulfide (Li2S).
“But the presence of a small amount of catalyst in the cathode makes all the difference,” Xu said. “A much different reaction pathway follows, one free of intermediate reaction steps.”
Key is the formation of dense nanoscale bubbles of lithium polysulfides on the cathode surface, which do not appear without the catalyst. These lithium polysulfides rapidly spread throughout the cathode structure during discharge and transform to lithium sulfide consisting of nanoscale crystallites. This process prevents the sulfur loss and performance decline in commercial-size cells.
In unlocking this black box around the reaction mechanism, the scientists employed cutting-edge characterization techniques. Analyses of the catalyst’s structure with the intense synchrotron X-ray beams at beamline 20-BM of the Advanced Photon Source, a DOE Office of Science user facility, revealed that it plays a critical role in the reaction pathway. The catalyst structure affects the shape and composition of the final product upon discharge, as well as the intermediate products. With the catalyst, nanocrystalline lithium sulfide forms upon full discharge. Without the catalyst, microscale rod-shaped structures form instead.
“Our team’s efforts could bring the U.S. one large step closer to a greener and more sustainable transportation landscape.” — Gui-Liang Xu, chemist in Argonne’s Chemical Sciences and Engineering division
Another vital technique, developed at Xiamen University, allowed the team to visualize the electrode-electrolyte interface at the nanoscale while a test cell was working. This newly invented technique helped connect changes at the nanoscale to the behavior of an operating cell.
“Based on our exciting discovery, we will be doing more research to design even better sulfur cathodes,” Xu noted. “It would also be worthwhile to explore whether this mechanism applies to other next-generation batteries, such as sodium-sulfur.”
With this the team’s latest breakthrough, the future of lithium-sulfur batteries appears brighter, offering a more sustainable and eco-friendly solution for the transportation industry.
An article on this research appeared in Nature. In addition to Xu, authors include Shiyuan Zhou, Jie Shi, Sangui Liu, Gen Li, Fei Pei, Youhu Chen, Junxian Deng, Qizheng Zheng, Jiayi Li, Chen Zhao, Inhui Hwang, Cheng-Jun Sun, Yuzi Liu, Yu Deng, Ling Huang, Yu Qiao, Jian-Feng Chen, Khalil Amine, Shi-Gang Sun and Hong-Gang Liao.
Other participating institutions include Xiamen University, Beijing University of Chemical Technology and Nanjing University. The Argonne research was supported by the DOE Office of Vehicle Technologies in the Office of Energy Efficiency and Renewable Energy.
About the Advanced Photon Source
The U. S. Department of Energy Office of Science’s Advanced Photon Source (APS) at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. The APS provides high-brightness X-ray beams to a diverse community of researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. These X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being. Each year, more than 5,000 researchers use the APS to produce over 2,000 publications detailing impactful discoveries, and solve more vital biological protein structures than users of any other X-ray light source research facility. APS scientists and engineers innovate technology that is at the heart of advancing accelerator and light-source operations. This includes the insertion devices that produce extreme-brightness X-rays prized by researchers, lenses that focus the X-rays down to a few nanometers, instrumentation that maximizes the way the X-rays interact with samples being studied, and software that gathers and manages the massive quantity of data resulting from discovery research at the APS.
This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
####
About DOE/Argonne National Laboratory
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America’s scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy’s Office of Science.
The U.S. Department of Energy’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit https://energy.gov/science.
For more information, please click here
Contacts:
Diana Anderson
DOE/Argonne National Laboratory
Copyright © DOE/Argonne National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Possible Futures
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Discoveries
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Automotive/Transportation
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








