Home > Press > How to split a water molecule
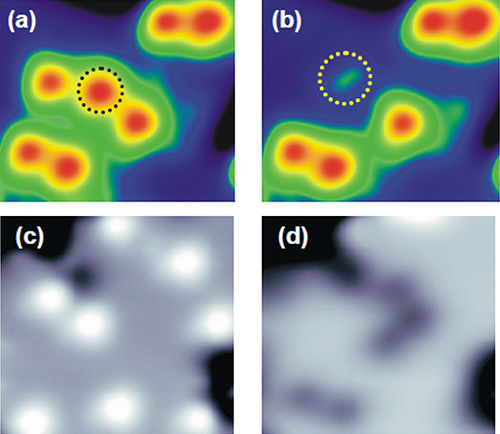 |
| Figure 3 STM images of water molecule before (a) and after (b) dissociation into OH, and before (c) and after (d) dissociation into O. |
Abstract:
A research team at RIKEN has succeeded for the first time in selectively controlling for reaction products in the dissociation of a single water molecule on an ultrathin film. The reaction, described in the April 19th issue of Nature Materials, opens the door to the creation of novel functional catalysts and applications in clean energy production.
How to split a water molecule
Tokyo | Posted on April 19th, 2010In recent years, the knowledge that materials exhibit novel properties at the nano-scale has driven a search for functional nano-materials with useful applications. Among these, ultrathin metal oxide films have attracted attention for their application in reaction catalysis, yet mechanisms underlying this catalytic role have remained unclear.
Using a scanning tunneling microscope (STM) at ultra-low temperatures, the research team explored the dynamics of single water molecules interacting with a film of magnesium oxide (MgO) several atoms in thickness. They discovered that by injecting tunnelling electrons into water molecules on the MgO surface, they could select between dissociation pathways: excitation of the molecule's vibrational states induced dissociation into hydroxyl (H + OH) (Figure 3 (a) and (b)), whereas excitation of its electronic states induced dissociation into atomic oxygen (O) (Figure 3 (c) and (d)).
The controlled dissociation of water molecules via selected reaction pathways presents unique opportunities in targeted catalysis, particularly in the production of hydrogen, a potential source of clean energy. While advancing our understanding of the dynamics of water molecules, the discovery also sets the stage for applications in the catalysis of more complex systems on insulating films.
####
About RIKEN
The mission of RIKEN is to conduct comprehensive research in science and technology (excluding only the humanities and social sciences) as provided for under the "RIKEN Law," and to publicly disseminate the results of its scientific research and technological developments. RIKEN carries out high level experimental and research work in a wide range of fields, including physics, chemistry, medical science, biology, and engineering, covering the entire range from basic research to practical application.
RIKEN was first organized in 1917 as a private research foundation, and reorganized in 2003 as an independent administrative institution under the Ministry of Education, Culture, Sports, Science and Technology.
For more information, please click here
Contacts:
Dr. Yousoo Kim
Surface and Interface Science Laboratory
RIKEN Advanced Science Institute
Tel: +81-(0)48-467-4073
Fax: +81-(0)48-462-4663
Ms. Tomoko Ikawa (PI officer)
Global Relations Office
RIKEN
Tel: +81-(0)48-462-1225
Fax: +81-(0)48-462-4715
Copyright © RIKEN
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
Thin films
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Environment
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








