Home > Press > Enzyme Detectives Uncover New Reactions, Products: Implications for engineering biofuels and plant-derived replacements for petro-chemicals
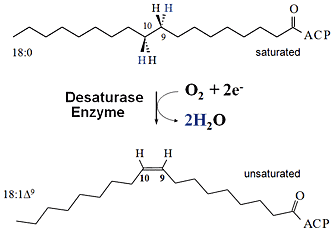 |
| Desaturation of Fatty Acids -- introducing a double bond turns bad fat into good fat. |
Abstract:
If your experiment doesn't go the way you expect, take a closer look something even more interesting may have happened. That strategy has led scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory to discover a fundamental shift in an enzyme's function that could help expand the toolbox for engineering biofuels and other plant-based oil products. The results will be published online the week of September 8, 2008, in the Proceedings of the National Academy of Sciences.
Enzyme Detectives Uncover New Reactions, Products: Implications for engineering biofuels and plant-derived replacements for petro-chemicals
UPTON, NY | Posted on September 8th, 2008 The Brookhaven scientists were trying to understand the factors that affect where carbon-carbon double bonds are placed in fatty acids, the building blocks of oils and fats, when they are "desaturated" that is, when a desaturase enzyme removes hydrogen from the carbon chain.
"Placing double bonds in different positions allows you to change the structure of the fatty acids to make products with different potential applications," explained Brookhaven biochemist John Shanklin, who led the research. The ultimate goal: engineering designer plant oils to be used as biofuels and/or raw materials to reduce the use of petroleum.
To try to change the position of a double bond, the Brookhaven team modified a desaturase enzyme, changing three of the 363 amino acids in its protein sequence. But when they tested the modified enzyme and looked for the expected product with its altered double-bond position, it wasn't there.
They could have moved on and made different amino acid changes to accomplish the initial goal. But Brookhaven research associate Edward Whittle was determined to figure out what was going on with the unusual result. "The substrate, or starting material, had been used up, so something was being produced substrates can't just disappear," Whittle said. "If it wasn't the product we were looking for, what was it?"
Whittle's detective work uncovered a remarkable discovery. Instead of producing a shift in double-bond position, the enzyme modification had yielded three completely new products two variations of a hydroxylated product called an allylic alcohol and a fatty acid containing two double bonds. "This was a profound shift in enzyme function," noted Shanklin, who has been working with modified enzymes for 15 years. "Usually you make changes very gradually, getting a few percent of a new product mixed with the original product. This was more like throwing a switch, making the change in function close to complete."
The discovery is also notable because the starting enzyme, like other soluble (membrane-independent) desaturases, can ordinarily perform only its one specified reaction desaturation. This is unlike desaturase enzymes that reside within the cell membrane, which appear to be more versatile, performing a range of reactions. The soluble and membrane enzymes, however, do share one key feature: both perform reactions that require the production of a highly reactive form of oxygen.
"Since both classes of enzymes produce activated oxygen, in theory the soluble enzymes, like their membrane counterparts, should be able to perform a variety of reactions as well," Shanklin said. "Our work demonstrates that this is indeed the case. Making small changes to the enzyme's amino acid sequence has unlocked the soluble desaturase's potential to facilitate a wider range of chemistry than has been seen before," Shanklin said.
The challenge is to figure out how these structural changes to the enzyme lead to the observed changes in reaction chemistry. Computer-generated models combining the known structure of the starting enzyme in conjunction with its new substrates are helping the scientists understand how the enzyme works. The next step is to obtain real 3-D crystal structures of enzyme-substrate complexes, using the National Synchrotron Light Source at Brookhaven Lab, to see how they match up with the predictions.
Analyzing the structures of soluble enzymes is much simpler than obtaining structures for membrane enzymes. So, in effect, this work is a fast-track approach for correlating structure with function, which should help scientists gain general mechanistic insights relevant to both classes of enzymes. "Understanding how nature has figured out how to do this very difficult chemistry, and how to control that chemistry," Shanklin said, "would be extremely satisfying from a purely scientific perspective. But applying this knowledge could have benefits for us all."
"Right now, the materials we use the plastics, foams, nylons have been limited by the structures of petroleum-based chemical feedstocks. But if we understand how to engineer designer desaturase-like plant enzymes, we can tailor-make feedstocks with optimal properties, instead of relying on the properties of preexisting raw materials," said Shanklin. "We'd no longer have to say, this is what we have so this is what we can make.' Instead, we could make the best feedstock for a particular application by designing the raw materials that will yield it."
This research was funded by the Office of Basic Energy Sciences within DOE's Office of Science, and by the Natural Sciences and Engineering Research Council of Canada. In addition to Shanklin and Whittle, the research team includes Amy Tremblay and Peter Buist of Carleton University in Ottawa, Canada.
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOEs Office of Science by Brookhaven Science Associates, a limited-liability company founded by Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization.
For more information, please click here
Contacts:
Karen McNulty Walsh
(631) 344-8350
or
Mona S. Rowe
(631) 344-5056
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Cambridge chemists discover simple way to build bigger molecules one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules one carbon at a time June 6th, 2025
![]() Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Videos/Movies
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








