Home > Press > New micromaterial releases nanoparticles that selectively destroy cancer cells
 |
Abstract:
Researchers at the Universitat Autňnoma de Barcelona (UAB), in collaboration with the Sant Pau Research Institute and the CIBER-BBN, have developed micromaterials made up only of proteins, capable of delivering over an extended period of time nanoparticles that attack specific cancer cells and destroy them. The micromaterials mimic natural secretory granules found in the endocrine system and were proven effective in mouse models of colorectal cancer.
New micromaterial releases nanoparticles that selectively destroy cancer cells
Barcelona, Spain | Posted on April 5th, 2024A team coordinated by Professor Antonio Villaverde from the Institute of Biotechnology and Biomedicine of the Department of Genetics and Microbiology, UAB, and with the participation of the Sant Pau Research Institute and the CIBER-BBN, has developed self-contained micromaterials made up only of proteins that are capable of delivering over an extended period of time the polypeptide that composes them. The technology used for the fabrication of these granules, patented by the researchers, is relatively simple and mimics the secretory granules of the human endocrine system. With regards to its chemical structure, it involves the coordination of ionic zinc with histidine-rich domain, an amino acid essential for living beings and therefore not toxic.
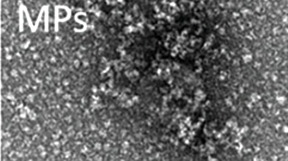
The new micromaterials developed by researchers are formed by chains of amino acids known as polypeptides, which are functional and bioavailable in the form of nanoparticles that can be released and targeted to specific types of cancer cells, for selective destruction.
The research team analyzed the molecular structure of these materials and the dynamics behind the secretion process, both in vitro and in vivo. In an animal model of CXCR4+ colorectal cancer, the system showed high performance upon subcutaneous administration, and how the released protein nanoparticles accumulated in tumor tissues.
“It is important to highlight that this accumulation is more efficient than when the protein is administered in blood. This fact offers an unexpected new way to ensure high local drug levels and better clinical efficacy, thus avoiding repeated intravenous administration regimens”, explains Professor Antonio Villaverde. "In the clinical context, the use of these materials in the treatment of colorectal cancer should largely enhance drug efficiency and patient’s comfort, while at the same time minimizing undesired side effects."
Participating in the research, conducted principally by UAB researcher Julieta M. Sánchez, were researchers from the UAB Department of Genetics and Microbiology, the UAB Institute of Biomedicine and Biotechnology (IBB-UAB), and the Oncogenesis and Antitumor Drugs team led by Professor Ramón Mangues of the Sant Pau Research Institute. Both Professor Antonio Villaverde and Professor Ramón Mangues form part of the CIBER network of Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN). Also participating in the study were the Protein Production Platform (Unit 1) and the Nanotoxicology Platform (Unit 18) of the Singular Infrastructure NANBIOSIS, and funding was received through several competitive research and technology transfer projects (including PID2019 -105416RB-I00/AEI/10.13039/501100011033, PDC2022-133858-I00, PID2022-136845OB-I00, CPP2021-008946, PI21/400), as well as intramural CIBER-BBN projects (VENOM4CANCER, NANOREMOTE and NANOSCAPE).
####
For more information, please click here
Contacts:
Media Contact
Octavi Lopez
Universitat Autonoma de Barcelona
Office: 34-935-813-301
Expert Contact
Antonio Villaverde
UAB
Office: +34 935813086
@UABBarcelona
Copyright © Universitat Autonoma de Barcelona
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Cancer
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
Synthetic Biology
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() The medicine of the future could be artificial life forms October 6th, 2023
The medicine of the future could be artificial life forms October 6th, 2023
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








