Home > Press > Led by Columbia Engineering, researchers build longest, highly conductive molecular nanowire: The 2.6nm-long single molecule wire has quasi-metallic properties and shows an unusual increase of conductance as the wire length increases; its excellent conductivity holds great promis
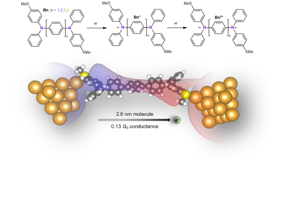 |
| Top: the two-step oxidation of the bis(triarylamines) molecular series. Bottom: the geometry of the highest conducting trimer (n=3) molecule in the molecular junction. Red and blue regions are artistic depictions on the coupling between the two edge states. CREDIT Liang Li/Columbia University |
Abstract:
As our devices get smaller and smaller, the use of molecules as the main components in electronic circuitry is becoming ever more critical. Over the past 10 years, researchers have been trying to use single molecules as conducting wires because of their small scale, distinct electronic characteristics, and high tunability. But in most molecular wires, as the length of the wire increases, the efficiency by which electrons are transmitted across the wire decreases exponentially.This limitation has made it especially challenging to build a long molecular wire--one that is much longer than a nanometer--that actually conducts electricity well.
Led by Columbia Engineering, researchers build longest, highly conductive molecular nanowire: The 2.6nm-long single molecule wire has quasi-metallic properties and shows an unusual increase of conductance as the wire length increases; its excellent conductivity holds great promis
New York, NY | Posted on July 8th, 2022Columbia researchers announced today that they have built a nanowire that is 2.6 nanometers long, shows an unusual increase in conductance as the wire length increases, and has quasi-metallic properties. Its excellent conductivity holds great promise for the field of molecular electronics, enabling electronic devices to become even tinier. The study is published today in Nature Chemistry.
Molecular wire designs
The team of researchers from Columbia Engineering and Columbia’s department of chemistry, together with theorists from Germany and synthetic chemists in China, explored molecular wire designs that would support unpaired electrons on either end, as such wires would form one-dimensional analogues to topological insulators (TI) that are highly conducting through their edges but insulating in the center.
While the simplest 1D TI is made of just carbon atoms where the terminal carbons support the radical states--unpaired electrons, these molecules are generally very unstable. Carbon does not like to have unpaired electrons. Replacing the terminal carbons, where the radicals are, with nitrogen increases the molecules’ stability. “This makes 1D TIs made with carbon chains but terminated with nitrogen much more stable and we can work with these at room temperature under ambient conditions,” said the team’s co-leader Latha Venkataraman, Lawrence Gussman Professor of Applied Physics and professor of chemistry.
Breaking the exponential-decay rule
Through a combination of chemical design and experiments, the group created a series of one-dimensional TIs and successfully broke the exponential-decay rule, a formula for the process of a quantity decreasing at a rate proportional to its current value. Using the two radical-edge states, the researchers generated a highly conducting pathway through the molecules and achieved a “reversed conductance decay,” i.e. a system that shows an increasing conductance with increasing wire length.
“What’s really exciting is that our wire had a conductance at the same scale as that of a gold metal-metal point contacts, suggesting that the molecule itself shows quasi-metallic properties,” Venkataraman said. “This work demonstrates that organic molecules can behave like metals at the single-molecule level in contrast to what had been done in the past where they were primarily weakly conducting.“
The researchers designed and synthesized a bis(triarylamines) molecular series, which exhibited properties of a one-dimensional TI by chemical oxidation. They made conductance measurements of single-molecule junctions where molecules were connected to both the source and drain electrodes. Through the measurements, the team showed that the longer molecules had a higher conductance, which worked until the wire was longer than 2.5 nanometers, the diameter of a strand of human DNA.
Laying the groundwork for more technological advancements in molecular electronics
“The Venkataraman lab is always seeking to understand the interplay of physics, chemistry, and engineering of single-molecule electronic devices,” added Liang Li, a PhD student in the lab, and a co-first author of the paper. “So creating these particular wires will lay the groundwork for major scientific advances in understanding transport through these novel systems. We’re very excited about our findings because they shed light not only on fundamental physics, but also on potential applications in the future.”
The group is currently developing new designs to build molecular wires that are even longer and still highly conductive.
####
For more information, please click here
Contacts:
Holly Evarts
Columbia University School of Engineering and Applied Science
Office: 212-854-3206
Cell: 347-453-7408
Copyright © Columbia University School of Engineering and Applied Science
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications July 5th, 2024
New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications July 5th, 2024
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
![]() New method cracked for high-capacity, secure quantum communication July 5th, 2024
New method cracked for high-capacity, secure quantum communication July 5th, 2024
![]() Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
![]() Atomic force microscopy in 3D July 5th, 2024
Atomic force microscopy in 3D July 5th, 2024
Physics
![]() New method cracked for high-capacity, secure quantum communication July 5th, 2024
New method cracked for high-capacity, secure quantum communication July 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
![]() Atomic force microscopy in 3D July 5th, 2024
Atomic force microscopy in 3D July 5th, 2024
![]() Aston University researcher receives £1 million grant to revolutionize miniature optical devices May 17th, 2024
Aston University researcher receives £1 million grant to revolutionize miniature optical devices May 17th, 2024
Possible Futures
![]() New method cracked for high-capacity, secure quantum communication July 5th, 2024
New method cracked for high-capacity, secure quantum communication July 5th, 2024
![]() Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
![]() Atomic force microscopy in 3D July 5th, 2024
Atomic force microscopy in 3D July 5th, 2024
Chip Technology
![]() New method cracked for high-capacity, secure quantum communication July 5th, 2024
New method cracked for high-capacity, secure quantum communication July 5th, 2024
![]() Diamond glitter: A play of colors with artificial DNA crystals May 17th, 2024
Diamond glitter: A play of colors with artificial DNA crystals May 17th, 2024
![]() Oscillating paramagnetic Meissner effect and Berezinskii-Kosterlitz-Thouless transition in cuprate superconductor May 17th, 2024
Oscillating paramagnetic Meissner effect and Berezinskii-Kosterlitz-Thouless transition in cuprate superconductor May 17th, 2024
Discoveries
![]() Efficient and stable hybrid perovskite-organic light-emitting diodes with external quantum efficiency exceeding 40 per cent July 5th, 2024
Efficient and stable hybrid perovskite-organic light-emitting diodes with external quantum efficiency exceeding 40 per cent July 5th, 2024
![]() A New Blue: Mysterious origin of the ribbontail ray’s electric blue spots revealed July 5th, 2024
A New Blue: Mysterious origin of the ribbontail ray’s electric blue spots revealed July 5th, 2024
![]() New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications July 5th, 2024
New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications July 5th, 2024
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
Announcements
![]() New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications July 5th, 2024
New organic molecule shatters phosphorescence efficiency records and paves way for rare metal-free applications July 5th, 2024
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
![]() New method cracked for high-capacity, secure quantum communication July 5th, 2024
New method cracked for high-capacity, secure quantum communication July 5th, 2024
![]() Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
![]() New method cracked for high-capacity, secure quantum communication July 5th, 2024
New method cracked for high-capacity, secure quantum communication July 5th, 2024
![]() Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
Searching for dark matter with the coldest quantum detectors in the world July 5th, 2024
![]() Atomic force microscopy in 3D July 5th, 2024
Atomic force microscopy in 3D July 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








