Home > Press > Bonding for color: Unscrambling the link between molecular bonding and distributing pigment leads to potential therapeutics for a rare skin disease
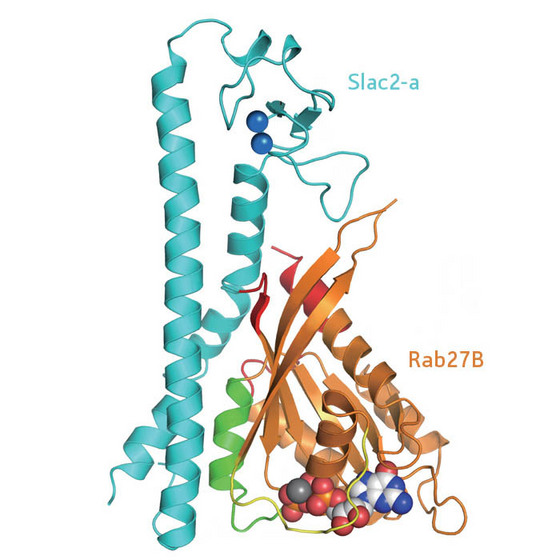 |
| Figure 1: Diagram of the structure of the Rab27B/Slac2-a complex. |
Abstract:
A RIKEN-led team of molecular biologists has determined the specific bonding leading to the formation of a protein complex involved in distributing pigment throughout the skin. Disruption of this membrane transport complex leads to the rare, lethal Griscelli syndrome for which there is no effective treatment. Patients show symptoms of albinism, suffer immunodeficiency, and typically die in early childhood. The work may stimulate the development of therapeutic drugs for the condition.
Bonding for color: Unscrambling the link between molecular bonding and distributing pigment leads to potential therapeutics for a rare skin disease
Japan | Posted on February 5th, 2009Members of the Rab protein family—of which there are more than 60 in humans—are thought to be essential to membrane trafficking, an important form of communication and distribution within cells. Rab proteins are typically bound to membranes either in an inactive guanine diphosphate form or an active triphosphate form which works through specific effector molecules to promote membrane trafficking.
The pigment melanin, which protects against radiation damage, is made and distributed within vesicles called melanosomes in skin color cells known as melanocytes. Rab27, which comes in two forms A and B, binds into a complex with the effector protein Slac2-a and myosin Va to transfer melanosomes onto actin filaments. The complex then transports the melanosomes along the filaments to where Rab27 uses another effector molecule to anchor them to the outer membrane of the cell.
Researchers from the RIKEN Systems and Structural Biology Center in Yokohama together with colleagues from Tohoku University were able to crystallize the Rab27B/Slac2-a complex and solve its structure using x-ray diffraction (Fig. 1). As active Rab27 proteins are notoriously difficult to crystallize, this was the first mammalian complex where the binding of such a protein with its effector molecule could be thoroughly investigated. The results were published recently in the journal Structure1.
The researchers found three contact regions between Rab27B and Slac2-a, of which only one was involved in specific recognition. Mutations affecting any of the several specific intermolecular hydrogen bonds in this region were fundamentally disruptive, and some of them led to Griscelli's syndrome. The group was able to verify the structure by taking another Rab protein, Rab3A, and engineering it to bind Slac2-a. The Rab3A amino acid sequence had to be altered by only four amino acid residues in the critical binding area to form the complex with Slac2-a.
"We are hoping that pharmaceutical companies will be able to use our structure as a basis for drugs which can be used to treat conditions like Griscelli's syndrome," says first author Mutsuko Kukimoto-Niino.
Reference
1. Kukimoto-Niino, M., Sakamoto, A., Kanno, E., Hanawa-Suetsugu, K., Terada, T., Shirouzu, M., Fukuda, M. & Yokoyama, S. Structural basis for the exclusive specificity of Slac2-a/melanophilin for the Rab27 GTPases. Structure 16, 1478-1490 (2008).
The corresponding author for this highlight is based at the RIKEN Systems and Structural Biology Research Center
####
For more information, please click here
Copyright © Riken
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








