Home > Press > How to halt immune cell activation
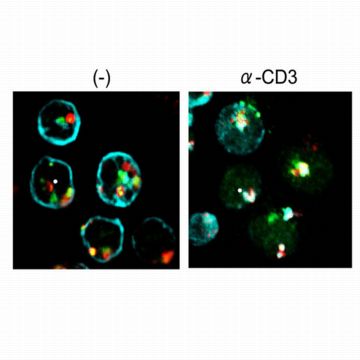 |
| Figure 1: Interaction between TCR, CD3 and LAPTM5. Left, CD3ζ (blue) is localized on the plasma membrane whereas LAPTM5 (green) and the lysosome-associated protein LAMP1 (red) are in the lysosomes in T cells before stimulation. Right, after TCR stimulation (α-CD3), CD3ζ moves to the lysosomal compartment where it co-localizes with LAPTM5 and LAMP1 and is degraded. Reproduced, with permission, from Ref. 1 © 2008 Elsevier Inc. |
Abstract:
A new study sheds light on the molecular machinery required for reining in cellular signals that, if unleashed, could result in pathological inflammation
How to halt immune cell activation
Japan | Posted on October 10th, 2008Researchers in Japan have identified part of the mechanism responsible for preventing prolonged—and potentially dangerous—activation of immune cells called T lymphocytes1. Each decorated with a unique surface receptor (TCR) capable of detecting pathogenic foreign proteins, T lymphocytes circulate throughout the body patrolling for invading microorganisms. Upon encounter with rogue proteins, TCRs trigger—via a complex of CD3 signaling proteins—intracellular events that orchestrate release of pro-inflammatory mediators called cytokines.
As unrestrained inflammation can cause tissue damage, the immune system exerts tight control over T lymphocyte activation. During healthy conditions, TCR and CD3 proteins are constantly internalized and released back to the lymphocyte surface; this ‘recycling' maintains a low level of TCR expression and thus a high ‘threshold' precluding unwarranted activation. After stimulation, however, TCRs and CD3 subunits are routed towards destructive intracellular compartments called lysosomes, where they are degraded as part of a signal ‘shut off' mechanism.
A team led by Ji-Yang Wang of the RIKEN Center for Allergy and Immunology in Yokohama sought to identify proteins underpinning this ‘fail safe' TCR signal termination process.
Having noted in previous experiments that expression of the lysosomal protein LAPTM5 is altered after TCR stimulation, the researchers tested whether LAPTM5 is involved in turning off TCR signals. They used genetic manipulation techniques to generate mutant mice in which the Laptm5 gene is not expressed. These Laptm5-deficient animals exhibited excessive T lymphocyte-driven responses to skin sensitization.
The team also found that, compared to normal T lymphocytes, LAPTM5-deficient T lymphocytes underwent more cell divisions, and released the cytokines interferon-γ and interleukin-2 more frequently after TCR stimulation. After activation, T lymphocytes lacking LAPTM5 expressed higher amounts of surface and intracellular TCR and a CD3 subunit, CD3ζ, than did wild-type T lymphocytes. Conversely, overexpression of LAPTM5 dampened CD3ζ expression.
TCR and CD3ζ proteins co-localized with LAPTM5 in lysosomes of activated T cells, and LAPTM5 physically interacted with CD3ζ (Fig. 1). These findings indicate that LAPTM5 might promote CD3ζ degradation by binding to and shuttling this protein to lysosomes.
Whether LAPTM5 cooperates with other lysosomal proteins to orchestrate CD3ζ destruction, and whether any human immune disorders are associated with mutations in Laptm5, remains to be determined.
LAPTM5 is the first lysosomal protein known to be specifically expressed in blood-generating (hematopoietic) cells. "In addition to its role in the negative regulation of TCR signaling, preliminary studies indicate that LAPTM5 may regulate the cell surface expression of additional immune receptors and may also function to prevent hematopoietic malignancies," says Wang.
Reference
1. Ouchida, R., Yamasaki, S., Hikida, M., Masuda, K., Kawamura, K., Wada, A., Mochizuki, S., Tagawa, M., Sakamoto, A., Hatano, M., Tokuhisa, T., Koseki, H., Saito, T., Kurosaki, T. & Wang, J.Y. A lysosomal protein negatively regulates surface T cell antigen receptor expression by promoting CD3ζ-chain degradation. Immunity 29, 33-43 (2008).
####
For more information, please click here
Copyright © Riken
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








