Home > Press > Imaging yields insights into 'nanomedicine' for cancer treatment
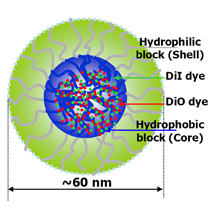 |
| Polymer micelle This illustration depicts a tiny drug-delivery particle called a polymer micelle, which has been used in “nanomedicine” techniques to treat cancer. Researchers at Purdue University have discovered a possible new pathway for anti-tumor drugs to enter cancer cells using the micelles and proposed how to improve the design of the tiny drug-delivery particles. (Weldon School of Biomedical Engineering, Purdue University) |
Abstract:
Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Förster resonance energy transfer imaging
Hongtao Chen*†, Sungwon Kim‡, Li Li*†, Shuyi Wang*§, Kinam Park*‡¶, and Ji-Xin Cheng*†¶
*Weldon School of Biomedical Engineering, †Department of Chemistry, and ‡Department of Pharmaceutics and Biomedical Engineering, Purdue University
It is generally assumed that polymeric micelles, upon administration into the blood stream, carry drug molecules until they are taken up into cells followed by intracellular release. The current work revisits this conventional wisdom. The study using duallabeled micelles containing fluorescently labeled copolymers and hydrophobic fluorescent probes entrapped in the polymeric micelle core showed that cellular uptake of hydrophobic probes was much faster than that of labeled copolymers. This result implies that the hydrophobic probes in the core are released from micelles in the extracellular space. Förster resonance energy transfer (FRET) imaging and spectroscopy were used to monitor this process in real time. A FRET pair, DiIC18(3) and DiOC18(3), was loaded into monomethoxy poly(ethylene glycol)-block-poly(D,L-lactic acid) micelles. By monitoring the FRET efficiency, release of the core-loaded probes to model membranes was demonstrated. During administration of polymeric micelles to tumor cells, a decrease of FRET was observed both on the cell membrane and inside of cells, indicating the release of core-loaded probes to the cell membrane before internalization. The decrease of FRET on the plasma membrane was also observed during administration of paclitaxel-loaded micelles. Taken together, our results suggest a membrane-mediated pathway for cellular uptake of hydrophobic molecules preloaded in polymeric micelles. The plasma membrane provides a temporal residence for micelle-released hydrophobic molecules before their delivery to target intracellular destinations. A putative role of the PEG shell in the molecular transport from micelle to membrane is discussed.
ABSTRACT
Fast Release of Lipophilic Agents from Circulating PEG-PDLLA Micelles Revealed by in ViWo Förster Resonance Energy Transfer Imaging
Hongtao Chen†, Sungwon Kim§, Wei He†, Haifeng Wang§, Philip S. Low†,|, Kinam Park‡,§,| and Ji-Xin Cheng*,†.‡,|
Department of Chemistry, Weldon School of Biomedical Engineering, Department of Pharmaceutics, and Oncological Sciences Center, Purdue University
Understanding the in ViVo behavior of nanoparticles is critical for the translation of nanomedicine from laboratory research to clinical trials. In this work, in ViVo Förster resonance energy transfer (FRET) imaging was employed to monitor the release of hydrophobic molecules from circulating poly(ethylene glycol)-poly(D,L-lactic acid) (PEGPDLLA) micelles. A lipophilic FRET pair (DiIC18 and DiOC18) was physically entrapped into micelle cores by mimicking the loading of hydrophobic drugs. The FRET efficiency was found significantly reduced within 15 min after intravenous injection, implying that DiIC18 and DiOC18 quickly escaped from the circulating micelles. FRET spectroscopy studies further demonstrated that R- and â-globulins were major factors for the observed fast release, while ç-globulins, albumin, and red blood cells played minor roles. These results provide useful information for developing blood-stable micelles to deliver hydrophobic drugs to the target site via prolonged circulation and extravasation from the vascular system.
Imaging yields insights into 'nanomedicine' for cancer treatment
WEST LAFAYETTE, IN | Posted on May 2nd, 2008Researchers at Purdue University have discovered a possible new pathway for anti-tumor drugs to kill cancer cells and proposed how to improve the design of tiny drug-delivery particles for use in "nanomedicine."
The synthetic "polymer micelles" are drug-delivery spheres 60-100 nanometers in diameter, or roughly 100 times smaller than a red blood cell. The spheres harbor drugs in their inner core and contain an outer shell made of a material called polyethylene glycol.
Purdue researchers showed for the first time how this shell of polyethylene glycol latches onto the membranes of cancer cells, allowing fluorescent probes mimicking cancer drugs to enter the cancer cells, said Ji-Xin Cheng, an assistant professor in the Weldon School of Biomedical Engineering and Department of Chemistry.
"This is an interesting new step in developing nanomedicine techniques in drug delivery," he said.
The research is being led by Cheng and Kinam Park, Showalter Distinguished Professor of Biomedical Engineering and a professor of pharmaceutics.
New findings are detailed in two research papers. One paper appears this week in Proceedings of the National Academy of Sciences, and another paper also will appear in May in the journal Langmuir.
The researchers used an imaging technique called Förster resonance energy transfer imaging, or FRET, to make two key discoveries: how fluorescent molecules mimicking the cancer drug paclitaxel enter tumor cells and how the micelles break down in the blood before they have a chance to deliver the drug to cancer cells.
A critical feature of micelles is that they combine two types of polymers, one being hydrophobic and the other hydrophilic, meaning they are either unable or able to mix with water. The hydrophobic core was loaded with a green dye and the hydrophilic portion labeled with a red dye.
Experiments showed that "core-loaded" fluorescent molecules mimicking the drug entered cancer cells within 15 minutes, suggesting a new drug-delivery pathway to kill tumor cells, Cheng said.
The fluorescent probes produced a green color on the membranes and a yellowish color inside the cells.
"So this technique provides a system to monitor in real time how well anti-cancer drug delivery is working," Cheng said.
Additional findings appearing in Langmuir, in research using mice, show specifically how the drug is released prematurely in the blood.
"We first proved that micelles are unstable in the blood, and then we answered why they don't remain intact," Cheng said.
The researchers also propose a possible way to fix the problem by "crosslinking," or reinforcing polymer strands in the micelles with chemical bonds made of two sulfur atoms. This reinforced structure might remain intact in the blood long enough to deliver the micelles to tumor sites, where they would biodegrade, Cheng said.
The researchers are the first to use FRET to study drug release from polymer micelles into a tumor cell.
Because micelles remain intact in water, researchers had thought the particles were stable in blood, but Canadian scientists in 2003 showed that the micelles are quickly broken down, releasing the drug into the blood.
"The reason is very simple," Cheng said. "Unlike water, blood has many components like surfactants and lipids and proteins that interact with the whole micelle structure. As a result, the micelles are unstable in blood and the drug is released too soon."
The Purdue researchers tested how stable micelles are in different blood components. Findings indicated that the micelles remained intact in red blood cells and components of blood plasma except for a class of plasma proteins called alpha and beta globulins, which caused the drug to be released.
"There could also be other blood components that cause the drug to be released, but our proposal of using crosslinking could prevent this from happening," Cheng said.
Future research may concentrate on creating micelles that remain intact longer in the blood by using the crosslinking.
The research has been funded by the Oncological Sciences Center in Purdue's Discovery Park, the National Science Foundation and the National Institutes of Health.
The research paper appearing this week in Proceedings of the National Academy of Sciences was written by graduate students Hongtao Chen and Li Li, and visiting scholar Shuyi Wang, post-doctoral research assistant Sungwon Kim, Park and Cheng. The paper appearing in May in Langmuir was written by Chen, Kim, and doctoral students Wei He and Haifeng Wang, Philip S. Low, the Ralph C. Corley Distinguished Professor of Chemistry and a researcher in Purdue's Oncological Sciences Center, Park and Cheng.
Note to Journalists: Electronic copies of the two research papers are available from Emil Venere, (765) 494-4709,
####
About Purdue University
Founded in 1869 and named after benefactor John Purdue, Purdue University began its journey with six instructors, 39 students and a mission to provide agriculture and mechanic arts education.
Student Body
System-wide enrollment of 69,098 students; West Lafayette enrollment of 38,712 students (fall 2005); students from 50 states and 130 countries.
For more information, please click here
Contacts:
Writer: Emil Venere, (765) 494-4709,
Sources: Ji-Xin Cheng, (765) 494-4335,
Kinam Park, (765) 494-7759,
Purdue News Service: (765) 494-2096;
Copyright © Purdue University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








