|
This issue of NanoNews-Now covers Nanomedicine. Editor Rocky Rawstern interviews J. Donald Payne of Nanospectra Biosciences, Sadik Esener (UCSD CCNE) and William Vine (NanoBioNexus), and Peter Searson of the Johns Hopkins Institute for NanoBioTechnology.
In our main article, Julian L. Zegelman, Director of Corporate Partnerships and Alliances at NanoBioNexus, writes an artice titled NanoBioNexus: a study in community building.
Next, the first of three interviews, this one with Sadik Esener (UCSD CCNE) and William Vine (NanoBioNexus) on the NanoTumor Center.
Following that are excerpts from several recent articles by the father of nanomedicine, Robert A. Freitas Jr.
Next up is Lynn Yoffee, of NanoBiotech News, with an article titled Nanomedicine and nano device pipeline surges 68%.
Contributing once again is Futurist Brian Wang, with his article titled On the way to Nanomedicine: Decisions and technology past and future.
Following Wang's article are two interviews, the first with J. Donald Payne of Nanospectra Biosciences, and the second with Peter Searson of the Johns Hopkins Institute for NanoBioTechnology.
In the 5th of 6 articles on Building The Winning Nano Venture Team, Bo Varga covers What investors and customers look for in start-up companies.
And last, two nanomedicine art pieces by Tim Fonseca.
Technology Monitoring Service
Helping form profitable partnerships
Nanotechnology Now, the leading Internet portal for nanotech information, provides a specialized service for businesses committed to bringing new technologies to the people and institutions that need them. We call it the Technology Monitoring Service.
What kinds of proprietary services and specialized information do we provide?
- A monitoring service, updated by a crew of professional investigators working closely with university researchers, administrators and attorneys involved in technology transfer.
- In addition to the Technology Monitoring Service, subscribers will receive our NanoTech Transfer Report and University Patent Database.
- Subscribers will also receive our bi-weekly NanoNews Custom, our "what you want - when you want it" solution to NanoNews.
Learn more here
|
|
|
Table of contents:
Opening Statement
Julian L. Zegelman
Esener/Vine
Robert A. Freitas Jr.
Lynn Yoffee
Brian Wang
J. Donald Payne
Peter Searson
Bo Varga
Nanomedicine Gallery
Quotes
News
Useful Links
Next Issue
Glossary
Editorial Calendar
About Us
Advertise
Contact
|
 |
|
 |
|
 |
|
 |
University Technology Transfer & Patents
Learn More |
 |
Only the news you want to read!
Learn More |
 |
|
 |
Full-service, expert consulting
Learn More |
|
Advertisement
|

|
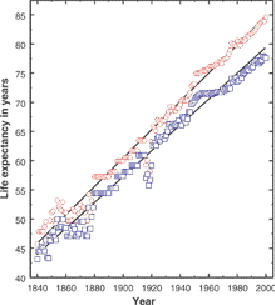
Live longer, better, with nanomedicine
Rocky Rawstern
Editor Nanotechnology Now
|
While there is speculation surrounding the time-frame and extent to which nanomedicine will change our lives, there is no doubt about the level of seriousness that major research groups are giving to medically-oriented nanoscale technologies, nor the amount of dollars being spent in an effort to change one of today's greatest killers (1) into an easily diagnosed and treated illness.
Witness the Mission Statement of the National Cancer Institute (NCI) Alliance for Nanotechnology in Cancer:
"To help meet the goal of eliminating suffering and death due to cancer, the National Cancer Institute (NCI), part of the National Institutes of Health, is engaged in efforts to harness the power of nanotechnology to radically change the way we diagnose, treat and prevent cancer.
The NCI Alliance for Nanotechnology in Cancer is a comprehensive, systematized initiative encompassing the public and private sectors, designed to accelerate the application of the best capabilities of nanotechnology to cancer.
Currently, scientists are limited in their ability to turn promising molecular discoveries into benefits for cancer patients. Nanotechnology can provide the technical power and tools that will enable those developing new diagnostics, therapeutics, and preventives to keep pace with today’s explosion in knowledge."
And this, from the NIH Roadmap for Medical Research:
"What if doctors could search out and destroy the very first cancer cells that would otherwise have caused a tumor to develop in the body? What if a broken part of a cell could be removed and replaced with a miniature biological machine? What if pumps the size of molecules could be implanted to deliver life-saving medicines precisely when and where they are needed? These scenarios may sound unbelievable, but they are the long-term goals of the NIH Roadmap's Nanomedicine initiative that we anticipate will yield medical benefits as early as 10 years from now."
This isn't an Apollo Moon Mission, nor is it a Manhatten Project, yet. It is, however, a concerted effort to - as stated above - "meet the goal of eliminating suffering and death due to cancer" and is being funded to the tune of millions of dollars per year (2) by the NIH, and many more millions by groups spread around the world. Someone is taking nanomedicine seriously, and the upshot is potentially and end to one of humankind's greatest scourges, cancer.
As you will read below, efforts are underway to meet the challenge of diagnosing and treating many of the diseases that currently limit us to a scant seven or eight decades of life.
I, for one, look forward to however many extra (and extra quality) years that nanomedicine will offer.
(1) The CDC puts the total killed by cancer in the U.S. in 2003 at 557,271, second only to heart disease at 696,947.
(2) Centers of Cancer Nanotechnology Excellence (CCNEs): Eight hubs over 5 years - $26.3 million for the first year. Cancer Nanotechnology Platform Partnerships: 12, 5-year R01 awards - $7 million for the first year. From the CCNE Fact Sheet link

|
NanoBioNexus: a study in community building
Julian L. Zegelman, Esq., Director of Corporate Partnerships and Alliances, NanoBioNexus
|
In the last thirty years, economic research has repeatedly demonstrated that successful development, growth and ultimate profitability of a new industry is intimately tied to the existence of active social networks serving to connect the otherwise autonomous players involved in the specific nascent industry.
While additional factors, such as high rate of technological innovation, timely commercialization of academic discoveries, and sufficient access to capital, are undoubtedly important to the success of a young industry, many of these aforementioned goals are readily accomplished through community building efforts designed to create sustainable interactive social networks within the industry.
Of particular interest is research done by Drs. Michael R. Darby (1) and Lynne G. Zucker (2), which emphasizes the fact that majority of biotechnology and nanotechnology innovation and commercialization takes place at a few locales within the United States (3). The narrow geographic distribution of nanotechnology activity can be explained in terms of existing local conditions fostering interaction between academia, investors, entrepreneurs, service providers, government, and the general public.
Although nanotechnology is not a single industry in itself but a collection of multidisciplinary technology platforms with broad applications across many fields, creation of localized community networks dedicated to identification, development, and commercialization of nanotechnology inventions remains important to overall success of any region attempting to benefit from the tremendous economic potential of nanotechnology.
Headquartered in Southern California's biotechnology powerhouse, NanoBioNexus (4) of San Diego, CA, is one such community network dedicated to increasing understanding of the issues surrounding nanotechnology and facilitating business opportunities for nanotechnology applications in the life sciences arena. NanoBioNexus is a non-profit, volunteer managed organization offering nanotechnology focused market research, consulting and business development services, in addition to monthly networking and educational programs delivered in the San Diego area. Through a series of monthly networking and educational forums combined with web-based outreaches, the organization connects with a broad target audience of business and legal professionals, venture capitalists, entrepreneurs, scientists, and members of the general public with an interest in nanobiotechnology.
NanoBioNexus was created in 2004 by Adriana Vela, a distinguished marketing executive with a keen interest in the life sciences, to fill an existing void in San Diego nanotechnology scene. Within two years from inception, we have attracted a devoted executive team, recruited committed industry supporters, produced monthly educational forums, launched a suite of professional consulting services for the benefit of our constituency, and were contracted to head the educational component at a regional Center of Cancer Nanotechnology Excellence.
NanoBioNexus's volunteer executive team unites accomplished professionals with such diverse yet highly complementary backgrounds as law, finance, marketing, science and medicine. Recent launch of consulting services enabled the organization to provide competent and competitively priced solutions to freshly minted nanotechnology entrepreneurs and established companies alike. NanoBioNexus consultants are often invited to provide expertise in the areas of technology due diligence, market research, executive recruiting, legal counseling, intellectual property management, business development, etc. As a non-profit organization looking to give back to the community it serves, NanoBioNexus is able to price its professional services below prevalent market rates.
Monthly forums produced by NanoBioNexus consist of both networking and educational components. At any given monthly forum, a networking reception taking place prior to the feature presentation gives attendees an opportunity to make new contacts in the area and meet other individuals active in the nanotechnology field. During the educational component, selected guest speakers deliver presentations on a particular topic of interest. Previously covered topics include nanotechnology applications to drug discovery and drug delivery, the future of nanotherapeutics, and commercialization of nanotechnology. Due to their increased popularity, the content of NanoBioNexus monthly forums is now available in the form of web-casts accessible through the organization's website.
NanoBioNexus's ability to educate and facilitate communication in the community for applications of nanotechnology in the life sciences was instrumental in its selection to head the educational component of the recently created Center of Nanotechnology for Treatment, Understanding, and Monitoring of Cancer ("NanoTUMOR"), one of only seven such Centers of Cancer Nanotechnology Excellence nationwide (5). Funded by the National Cancer Institute and headed by the University of California San Diego, the Southern California based NanoTUMOR center is a research partnership bringing together the University of California San Diego, Moores UCSD Cancer Center, the Burnham Institute for Medical Research, NanoBioNexus, and University of California campuses at Irvine, Riverside, and Santa Barbara. The focus of the center is to develop multifunctional oncology nanoplatforms capable of selectively targeting tumors and delivering new generation of cancer therapeutics.
The role of NanoBioNexus within the NanoTUMOR center is to coordinate education programs in nanotechnology and its applications to oncology, enhance interaction between the member institutions, and educate community healthcare providers and the general public about the nanotechnology applications to cancer treatment.
Two years later, NanoBioNexus begins to see some of its impact on the San Diego community. Long recognized for its vibrant biotechnology landscape, the city is now beginning to attract more nanotechnology start-ups interested in leveraging existing financial, personnel and scientific resources San Diego is able to provide. Not surprisingly, NanoBioNexus is repeatedly invited to serve as a conduit between incoming start-ups and the established local community.
As part of its commitment to global community building, NanoBioNexus is often called upon to share its lessons with communities outside of San Diego that become interested in starting local nanotechnology groups. In the past six months, members of the organization's executive team participated in dialogues with international colleagues from Sweden, Canada, Mexico, Israel and Russia. Some of the recent outreach efforts involved trips to Canada and Mexico by Adriana Vela, the founder and chair of NanoBioNexus. In the age of globalization and increased cooperation, NanoBioNexus stands ready to help other communities replicate its effort to jumpstart local nanotechnology initiatives.
1. Professor of Policy and Director of the John M. Olin Center for Policy at UCLA Anderson School of Management
2. Professor in the Departments of Sociology and Social Studies at UCLA
3. Intellectual Capital and the Birth of U.S. Biotechnology Enterprises. NBER Working Paper Series. Working Paper No: 4653, February 1994. Lynne G. Zucker, Michael R. Darby, and Marylinn B. Brewer. Grilichesian Breakthroughs: Inventions of Methods of Inventing and Firm Entry in Nanotechnology. NBER Working Paper Series. Working Paper No: 9825, July 2003. Michael R. Darby and Lynne G. Zucker.
4. www.nanobionexus.com
5. link
|
Julian L. Zegelman, Esq.
Zegelman is the Partnerships and Alliances Director at NanoBioNexus, a San Diego, CA based non-profit organization devoted to advancing applications of nanotechnology in the Life Sciences. He also practices Intellectual Property and Corporate Law at Catalyst Law Group, a boutique San Diego law firm serving the biotechnology and pharmaceutical industries. Julian is a graduate of the University of Minnesota Law School, where he served as a staff member of the Minnesota Intellectual Property Review, a publication devoted predominantly to intellectual property matters. Julian completed his undergraduate education at the University of California, San Diego (UCSD), where he was awarded a Bachelor of Science in biochemistry and chemistry with a minor in political science.
About NanoBioNexus
NanoBioNexus is a non-profit, volunteer-managed organization that showcases applications of nanotechnology in the Life Sciences. As an organization, its mission is to provide a community service by building awareness and understanding of nanotechnology and by fostering business opportunities in the application of nanotechnology in the life sciences. This is accomplished through market research as well as networking and partnering programs delivered in the San Diego area with outreach to all of Southern California and beyond.
Our vision is to be recognized as the leading group of economic observers and business arbiters for the nanobiotechnology field. The group's target audience is business and legal professionals, venture capitalists, entrepreneurs, academia, and members of the general public with an interest in nanobiotechnology.
|
NN: Please talk about the UCSD Center of Cancer Nanotechnology Excellence (AKA NanoTUMOR Center); how it came to be; its goals; and current status.
Esener: There was a considerable amount of ongoing work in various aspects of nanotechnology at different locations at the UCSD campus as well as our partner institutions. The NanoTUMORCenter has enabled us to bring researchers from various disciplines -such as chemists, physicists, engineers, biologist and oncologist - together with the aim of applying nanotechnology towards the understanding, treatment and monitoring of cancer. The center now supports more than 35 co-investigators. We now have a central laboratory dedicated to cancer nanotechnology at the Moores Cancer Center with satellite laboratories distributed at the UCSD campus including the CalIT2 building and at our partner institutions.
NN: The National Cancer Institute (NCI) awarded UCSD $3.9 million for the first year of a five-year, $20 million cancer-nanotechnology platform partnership. In general, how will the $3.9 million be allocated?
Esener: The $3.9 million per year funding is distributed among six projects and the related core facilities. Each project is developing a different aspect of cancer nanotechnology that will create platforms for more powerful and selective cancer therapy when integrated together. Each project will work to advance our tools for earlier detection of cancer, and our understanding of the progress of the disease.
NN: Which institutions are participating in the NTC? What expertise do they bring?
Esener: In addition to UCSD, our partner institutions include UCSB, which is well known for its capabilities in nanotechnology, and Burnham Institute, where different types of targeting peptides have been developed and tested. In addition, investigators from UC-Riverside and UC-Irvine bring certain specific expertise to the program while NanoBioNexus manages our educational activities.
NN: In general what types of research projects are planned over the five years of the program and what are their goals?
Esener: One focus of the UCSD effort will be to develop smart hierarchical delivery platforms about the size of a red blood cell. These "mother ships" would move through the body and target specific tumor cells or the blood vessels that feed them. After arriving at their destinations, the mother ships would release their payload nanoparticles, which could be designed to help image tumors, enter cells and perform measurements, and deliver therapies. Chemists at UCSD, together with materials scientists at the University of California, Santa Barbara nanofabrication facility, will synthesize nanoparticles that will be coated with "biolinkers," molecules developed at the Burnham Institute to make the particles attach to specific types of tumor cells.
NN: On April 4, 2006, NanoBioNexus announced that they will head the educational component of the NanoTUMOR Center. Why is an Educational Core needed?
Vine: The National Cancer Institute requested that each of the eight Centers of Cancer and Nanotechnology Excellence (CCNE) create community outreach and internal educational programs in nanotechnology and cancer. Nanotechnology is so new that scientists and physicians as well as the general public have a thirst to learn more. Certainly, they are curious about how nanotechnology can help people with cancer. Finally, we want to replace the misinformation that creates anxiety with the facts so that the full benefits of nanotechnology are realized. Thus, the job of the Educational Core is to plan and execute the corresponding programs
NN: Who are the intended "users" of the educational materials?
Vine: We want to target the general public because everyone, directly or indirectly, is affected by cancer. We also want to establish close ties to local health care providers and research professional so that they are aware of new discoveries and potential therapeutics. Finally, we shall catalyze education, communication and cooperation within the NTC by our efforts.
NN: Why was NanoBioNexus chosen by the NanoTUMOR Center to Implement the Educational Core?
Vine: NBN has an expertise in teaching the public, including scientists, about both nanotechnology and biotechnology. This combination of educational expertise in the nanotech/biotech space and in teaching both the general public as well as scientists is unusual.
NN: What are the goals of the educational efforts?
Vine: Our goals are tailored to audiences: For the general public, we want to provide realistic information about the emerging field of nanotechnology and its current and potential beneficial impacts upon cancer diagnosis and treatment. For the professionals of the community, we need to provide relevant and realistic technical information in an attractive format. For the members of the NTC, our goals are not only to provide technical information about nanotechnology and cancer but also to facilitate the research of the NTC through enhanced communication and networking.
NN: What types of projects will NanoBioNexus undertake?
Vine: Our projects are tailored to the specific audience and critical for success. For the general public we will create a website full of useful information on nanotechnology and cancer, provide a calendar of local events on these important subjects and encourage local media to produce timely and factual content. Our website will also contain more technical information for health care providers and professionals in the nanotech/biotech space. NanoBioNexus also seeks to establish personal ties to all professionals. We plan to visit local hospitals to lecture on cancer and nanotechnology in a way that is relevant for health care providers. Likewise, many of our seminars, which focus on research in nanotechnology and cancer, will be open to interested professionals.
We have an extensive list of projects specifically for the NTC. We shall plan and host multiple seminars, the staple of communication in the scientific community. We will add occasional workshops, intensive daylong meetings designed for extensive discussion, development and coordination. Then there is the private website of the NTC, which will have multiple tools for education and communication. The tools include recordings of seminars for later reference, FAQ's, calendar of events, a moderated forum, an annotated bibliography, etc.
We look forward to the full implementation of these and other projects, which will occur over the next few years. We are very excited about the opportunity to expose all to this exciting research and its vast potential to treat cancer.
NN: To date, what are the most promising nanoplatforms for cancer diagnostics and treatment? How do they work?
Esener: It is far too early to even attempt to answer this question. However, it is well accepted today that drug delivery via nanotechnology offers great potential and has significant advantages over more conventional techniques. The advantages include:
- Highly selective targeted delivery via blood vessels to specific desired locations using, for example, peptides developed at Burnham Institute for targeting
- Reduce undesired side effects
- Reduce drug quantities while increasing effectiveness by delivering the treatment right to the tumor
- Delivery of multiple drugs regardless of their pharmacokinetic compatibility
NN: Looking out 10 years, what are your hopes regarding medical diagnostics and treatments stemming from our understanding of the nanoscale?
Esener: Our hope in general is to be able to reduce suffering and death caused by cancer and to significantly improve the quality of life for cancer patients and their families. More specifically, we hope to develop platforms that can detect cancer at its earliest stage. We hope to be able to monitor and treat residual cancerous cells after treatment and be able to provide treatment with high specificity and efficiency eliminating side effects and the need to perform open surgery to remove tumors.
|
Sadik Esener, Ph.D.
Professor Esener is an internationally known expert in photonics and opto-electronics, and he has been closely involved with five startup companies based on technology developed in his laboratories. Professor Esener co-founded San Diego-based Nanogen, Optical Micro-Machines, Parallel Solutions, Genoptix, and Call/Recall Inc.
His research interests include light modulation, detection, and amplification, heterogeneous integration of optoelectronic components, optical data storage, optical interconnects and related computing architectures, and biophotonics as applied to gene chips. Esener is a pioneer in the fields of free-space optical interconnects, parallel access volumetric optical data storage, DNA-assisted heterogeneous integration and optical cell sorting, and holds many patents inthese areas. Esener's research team is working on diverse projects pushing the limits of the state of the art. They include active and passive photonic device processing and hybrid integration techniques; photonics sub-systems assembly such as optically interconnected Fast Fourier Transform accelerator boards; and parallel light tweezer systems for handling and characterization of biological entities.
Esener joined the UCSD faculty in 1987, after receiving his Ph.D. in Applied Physics and Electrical Engineering from UCSD the same year. He leads UCSD's OptoElectronic Computing Group, and is the director of: the DARPA-funded multi-university Center for Chips with Heterogenously Integrated Photonics (CHIPS); the 3D-Opto-Electronic Stacked Processors industry/university consortium; and the Fast Read-out Optical Storage (FROST) Industry consortium. He has authored more than 100 journal publications and 200 conference abstracts. Esener is a member of IEEE, OSA, and SPIE.
Dr. William Vine
Vine is Director of Strategic Programs at NanoBioNexus and PI of the Educational Core of the NanoTUMOR Center. He has been a Senior Scientist at Arena Pharmaceuticals, where he implemented SMART (Sensitive Mass Assisted Receptor Technology), which he had previously invented. SMART integrates high sensitivity mass spectrometry with endocrinology to discover new hormones. Previously, he was Associate Director of Physiology at Amylin Pharmaceuticals, where he made critical contributions to their drug pipeline, including Symlin and BYetta. He has been a member of the Faculty at Yale University, the University of Minnesota, and the University of Cincinnati, where he directed the Clinical Chemistry Laboratory as an Associate Professor. He earned his MD, PhD and AB from Washington University; has clinical expertise in Chemical Pathology, having completed a residency in Laboratory Medicine at Yale, New Haven Hospital; and completed an Internship in Pediatrics at Cardinal Glennon Hospital. He has one patent and over 40 peer-reviewed publications to his credit.
Additional Links:
CCNE Fact Sheet
Biographies - CCNEs
Questions and Answers: CCNE
(Ed.'s note: Special thanks to Sandra Kay Helsel and the team at NanoBioNexus for all their help putting this interview together.)
|

|
Robert A. Freitas Jr., Author, Nanomedicine Vol.'s I and IIA, Senior Research Fellow, Institute for Molecular Manufacturing.
|
The following are excerpts from Dr. Freitas' most-recently published articles, reprinted here with his permission.
An exciting revolution in health care and medical technology looms large on the horizon. Yet the agents of change will be microscopically small, future products of a new discipline known as nanotechnology. Nanotechnology is the engineering of molecularly precise structures - typically 0.1 μm or smaller - and, ultimately, molecular machines.
Nanomedicine is the application of nanotechnology to medicine. It is the preservation and improvement of human health, using molecular tools and molecular knowledge of the human tools and molecular knowledge of the human structured nanoparticles such as dendrimers, carbon fullerenes (buckyballs) and nanoshells to target specific tissues and organs. These nanoparticles may serve as diagnostic and therapeutic antiviral, antitumor or anticancer agents. But as this technology matures in the years ahead, complex nanodevices and even nanorobots will be fabricated, first of biological materials but later using more durable materials such as diamond to achieve the most powerful results.
The greatest power of nanomedicine will emerge, perhaps in the 2020s, when we can design and construct complete artificial nanorobots using rigid diamondoid nanometer-scale parts like molecular gears and bearings. These nanorobots will possess a full panoply of autonomous subsystems including onboard sensors, motors, manipulators, power supplies, and molecular computers.
The ability to build complex diamondoid medical nanorobots to molecular precision, and then to build them cheaply enough in sufficiently large numbers to be useful therapeutically, will revolutionize the practice of medicine and surgery.
From "Nanotechnology, Nanomedicine and Nanosurgery (Invited Editorial)," Intl. J. Surgery 3(December 2005):1-4. (PDF)
Massively parallel assembly is the key to the economic viability of molecular manufacturing.Biology provides perhaps the best example of the power of massive parallelism in assembly, such as polysomes in living cells (multiple ribosomes translating a single mRNA strand simultaneously).The difference between serial and parallel processing is similarly crucial in molecular manufacturing, where the basic parts are very small. If a typical molecularly precise simple component is 1 nm3 in volume, then to manufacture a 1 cm3 volume of molecularly precise product requires the assembly of 1000 billion billion (1021) individual simple molecular components - even at a 1 GHz operating frequency, serial atom-by-atom manufacturing of a single object would take many thousands of years, clearly not economically viable.But with parallel manufacturing, vast numbers of molecular components can be processed simultaneously, reducing batch processing times to days, hours, or even less.At least two such techniques for performing massively parallel positional assembly have been identi.ed: (1) massively parallel manipulator arrays and (2) self-replicating systems.
From "Current Status of Nanomedicine and Medical Nanorobotics (Invited Survey)," J. Comput. Theor. Nanosci. 2(March 2005):1-25 (PDF)
Our near-term ability to structure materials and devices at the molecular scale brings enormous immediate benefits and will revolutionize the research and practice of medicine. Early theoretical and experimental studies of the biocompatibility of nanomaterials and advanced nanodevices have begun . Taking Feynman’s long-term vision of medical nanorobots to heart, our present knowledge tells us that these things violate no known laws of physics, chemistry, biology, or engineering. Complex issues relating to future US Food and Drug Administration approval of nanomedical materials, devices, and even the possibility of medical nanorobots are already being addressed in mainstream legal journals [82,83]. One hopes that our society will be able to muster the collective financial and moral courage to allow such extraordinarily powerful medicine to be deployed for human betterment, with due regard to essential ethical considerations.
From "What is Nanomedicine?” Nanomedicine: Nanotech. Biol. Med. 1(March 2005):2-9 (PDF)
Is the advent of, and mass availability of, desktop personal nanofactories (PNs) likely to cause deflation (a persistent decline in the general prices of goods and services), inflation (a persistent general price increase), or neither?
A definitive analysis would have to address: (1) the technical assumptions that are made, including as yet imprecisely defined future technological advances and the pace and order of their introduction; (2) the feedback-mediated dynamic responses of the macroeconomy in situations where we don’t have a lot of historical data to guide us; (3) the counter-leaning responses of existing power centers (corporate entities, wealthy owners/investors, influential political actors, antitechnology-driven activists, etc.) to the potential dilution of their power, influence, or interests, including their likely efforts to actively oppose or at least delay this potential dilution; (4) legal restrictions that may be placed on the widespread use of certain technological options, for reasons ranging from legitimate public safety and environmental concerns to crass political or commercial opportunism; (5) the possibility (having an as yet illdefined probability) that nanotechnology might actually “break the system” and render conventional capitalism obsolete (much as solid state electronics obsoleted vacuum tubes), in which case it is not clear what new economic system might replace capitalism; and (6) the changes in human economic behavior that may result when human nature itself may have changed.
Conclusions
Deflationary forces driven by advances in molecular manufacturing (MM) can be opposed by inflationary forces competently initiated by governmental monetary authorities. This allows the two forces to remain roughly in balance, with the incremental inflation at the general price level remaining close to zero as PNs are introduced. Since an MM-rich economy will be dominated by services and information, not goods, our expectation is that the prices of services and information might rise very slightly as the prices of PN-manufactured goods falls significantly. For example, if services and information comprise 95% of the economy and goods are only 5% of all sales, then a deflationary –20% decline in the prices of goods can be largely offset by an inflationary rise of just +1% in the prices of services and information.
From Economic Impact of the Personal Nanofactory (PDF)
Readers who would like to learn more are encouraged to read the following articles:
Microbivores: Artificial Mechanical Phagocytes using Digest and Discharge Protocol
This paper presents a theoretical nanorobot scaling study for artificial mechanical phagocytes of microscopic size, called "microbivores," whose primary function is to destroy microbiological pathogens found in the human bloodstream using a digest and discharge protocol.
Theoretical Analysis of Diamond Mechanosynthesis. Part III. Positional C2 Deposition on Diamond C(110) Surface Using Si/Ge/Sn-Based Dimer Placement Tools (PDF)
This paper reports that the most-studied mechanosynthesis tooltip motif (DCB6Ge) successfully places a C2 carbon dimer on a C(110) diamond surface at both 300K (room temperature) and 80K (liquid nitrogen temperature), and that the silicon variant (DCB6Si) also works at 80K but not at 300K. Maximum acceptable limits for tooltip translational and rotational misplacement errors are reported in the paper. Over 100,000 CPU hours were invested in this study. The DCB6 tooltip motif, initially described at a Foresight Conference in 2002, was the first complete tooltip ever proposed for diamond mechanosynthesis and remains today the only tooltip motif that has been successfully simulated for its intended function on a full 200-atom diamond surface.
According to data compiled in the NanoBiotech News 2006 Nanomedicine, Device & Diagnostic Report, 130 nanotech-based drugs and delivery systems and 125 devices or diagnostic tests have entered preclinical, clinical, or commercial development, meaning the clinical pipeline has grown 68% since 2005.
"What we're seeing is a growing community of nanobiotech drug and device developers who are digging in their heels -- and surviving," says Lynn Yoffee, associate publisher of NanoBiotech News, which produced the 2006 Nanomedicine, Device & Diagnostic Report. "Along with that comes the advance of numerous product candidates marching beyond concept well into trials, ever closer to market. The industry is experiencing an evolution similar to what we saw in biotechnology, but the nanobiotech developers are putting together therapies and diagnostics with an even more astonishing 'wow' factor." Some of those promising products include:
- A nanoviricide for avian flu
- Nano-based coatings for medical implants that will permit safe magnetic resonance imaging
- A multifunctional nano device that selectively binds to cancer tumor cells and destroys them
"Although we keep a very close eye on the progress of drug candidates, we know the most immediate impact of nanotechnology in health care will be seen in earnest within the next couple of years in the form of medical devices. It's less complicated to get them developed and through the regulatory process," says Yoffee.
Big pharmas sit on the sidelines
Even with big pharma companies largely sitting on the sidelines, start-up companies are surviving and even thriving and new start-ups are emerging nearly every month. And the U.S. government added financial muscle to nanobiotech development in 2005, with major capital infusions through the National Cancer Institute's Alliance for Nanotechnology in Cancer and the National Institutes of Health's Program of Excellence in Nanotechnology.
The U.S. remains the leader in terms of the shear number (75%) of nano-based medical products in development, and of the 25% of drug and device candidates being developed outside of the U.S., Canada, Australia and Israel are working on 43% of the total 63 drugs and devices in the works around the world.
A plethora of new deals brewed in 2005. Nearly a third (30%) of all products are under development as part of collaborations or licensing deals, a trend similar to the biotechnology industry evolution.
A weed-out of start-ups
But during tough markets, only the top deals attract capital, says Douglas W. Jamison, president of New York venture capital firm Harris & Harris Group, Inc. (NASDAQ:TINY). When the market opens up, marginal companies also receive funding -- not necessarily a positive event for the market but certainly good news for start-up companies. From a capitalization standpoint, the biggest news during 2005 was the introduction of $20 million series A financings, which allowed companies to move their technologies into phase II clinical trials, Jamison says. But without new players coming into the nanobiotech market, the same investors are putting money into these deals.
Consequently, he expects to see fewer early stage deals in 2006 and a corresponding weed-out of nanobiotech start-ups.
"This could be the winnowing year for nanobiotech," Jamison says. "The cream will rise, and others will fail to receive second and third rounds of funding. In fact, that's already starting to occur."
|
Lynn Yoffee
Yoffee is associate publisher of National Health Information LLC (NHI) and NanoBiotech News, a weekly intelligence service covering the development of nanomedicines and nanomedical devices. She is the former senior managing editor of BioWorld Today, the newspaper of record for the biotechnology industry (www.bioworld.com). She can be reached at lyoffee@nhionline.net or (404) 607-9500.
NHI publishes a variety of monthly newsletters and special reports offering practical advice and detailed information on how to succeed under managed care. Each one of our publications is written to help you meet the challenges you face today -- from negotiating profitable contracts, to reducing utilization and cutting costs, to implementing demand and disease management programs.
|

|
On the way to Nanomedicine: Decisions and technology past and future
By Brian Wang
|
By Brian Wang, June 2006
Nanomedicine is the preservation and improvement of human health using molecular tools and molecular knowledge of the human body (1). The full realization of nanomedicine would require a robust form of molecular manufacturing. Molecular manufacturing is programmable control of precise molecular assembly that is highly scalable, but years - some say decades - in coming. However, a great deal could also be accomplished with advanced MEMS (2) (Micro-Electro-Mechanical Systems), genetics, bioengineering and synthetic biology. Nanomedicine will eliminate virtually all common diseases of the 20th century, virtually all medical pain and suffering, and allow the extension of human capabilities, most especially our mental abilities.
This paper will show that nanomedicine is a perfectly reasonable technological expectation. It will review some other powerful and nearer technologies for accomplishing some of the goals of nanomedicine. It will also review some of the arguments against medical technologies that could radically lengthen human lifespans. The case will be made that society should pursue better medical technologies and that we will be able to successfully adapt to increased lifespan and better health.
Nanomedicine and life extension are not crazy or impossible technologies
An example of a prospective nanomedicine system is the respirocyte, an artificial red blood cell. As envisioned, a respirocyte could transport oxygen 236 times more efficiently than red blood cells.
Nanomedicine capabilities are not far fetchedm and many goals could be achieved with other technical approaches. There are a lot of currently available blood substitutes (3). Red blood cells were modified in 2005 with magnetic beads that created an artificial tail. The artificial tail provided motion at one tenth the speed of sperm (4).
A group of NASA-funded bioengineers at the Universities of Pennsylvania and Minnesota have created double-walled artificial cells, called Polymersomes, that can potentially float through the bloodstream loaded with cargo, such as cancer-zapping drugs, imaging agents, and yes, extra oxygen (5).
The development of cell modification and small (less than 100nm) polymer spheres shows that creating a more complex device like a respirocyte is a difficult but achievable objective.
Aubrey De Grey has created a plan called SENS (6) (Strategies for Engineered Negligible Senescence) which has targeted seven factors that seem to be major components of aging. His basic argument is that "the SENS strategy is not to interfere with metabolism per se, but to repair or obviate the accumulating damage and thereby indefinitely postpone the age at which it reaches pathogenic levels." Nanomedicine seems likely to be able to help complete the aspects of SENS that are unsolved when nanomedicine arrives. If the seven factors of SENS are resolved, what will that mean for human lifespan? We do not know for sure, but it seems reasonable that it would be helpful.
Why make hasty assumptions about limits to health and lifespan?
Many people make hasty assumptions that there is a fixed maximum human lifespan.
According to a longevity study conducted by John Wilmoth ([S. J. Olshansky, B. A. Carnes, and A. Désesquelles, Science 292, 1654 (2001)] (7)) , a UC Berkeley associate, the "oldest age at death for humans has been rising for more than a century and shows no signs of leveling off." Wilmoth and fellow colleges from the United States and Sweden researched the national death records in Sweden and found an increase in the average maximum lifespan each year since 1861. This finding calls into question the 120-year lifespan limit (8).
People take the longest recorded human lifespan - that of Jeanne Calment, 122 years and 164 days - and then equate that as a fixed maximum. Before Jeanne Calment, the number was lower, at 110 or 120. Should Jeanne Calment have been euthanized at 115 years of age, before her longevity broke one of the prior records? Did her extra years take away from the dignity of other people who lived shorter lives?
Rational planning and control of medical research has flawed foundations
Rational planning and control of research is difficult, because we do not really have a good handle on what works with procedures that are already commonly used, and we have less of an idea about how well proposed research will succeed.
As shown in a recent Businessweek magazine article (9), the actual state of affairs in medicine is that we do not really know how well today's medical treatments work. This lack of certainty speaks against using some form of centralized planning to fund only what people think will work now. This method would prevent the funding of research, which could be surprisingly successful.
Many advanced medical technology are being opposed for non-technical reasons
Leon Kass, Chairman of the President's Council on Bioethics, and others argue for death by denying better technology. Arguing for death by enforced age limits or slaughtering the elderly are clearly moral non-starters. However, going at the issue of encouraging death indirectly is still extremely wrong. (Leon Kass, L'Chaim and Its Limits: Why Not Immortality? (10))
I will summarize Kass's argument for limited lifespans and my own rebuttals.
Kass asks "how might our finitude be good for us?" He offers four benefits.
First among which is interest and engagement. Kass asks: If the human life span were increased even by only twenty years, would the pleasures of life increase proportionately?
My answer: This is not a requirement. What we enjoy we will continue to enjoy. I do not affix a percentage to my enjoyment in life. The question is useless.
Second, seriousness and aspiration. Kass asks: Could life be serious or meaningful without the limit of mortality?
My answer: Day to day life would be the same for the first 40 years. The years from 50 to120 would be changed, to be similar to the condition from when the person was 30-40. It would not be a repeat of those years, but a rich and useful prolongation of that period. There are serious people who are 30-40 and even younger. The range of seriousness does not change with age. They have as many aspirations as those who are declining with age. Statistically, people will still die, but would no longer have the certainty of the decline in capacity and death from aging.
Most people do not spend more than a fraction of 1% of their time contemplating death from aging or factoring it into decisions and feelings. Most people do not consider death in any form more than 1% of the time. Most people do not plan for retirement, a widely expected phase of life before death. Why should one expect the loss of a consideration that is such a small percentage to have such an overweight impact? I say that it would not.
A third matter, beauty and love. Kass asserts: Someone who is immortal would not be able to love another as much because they would not have the fear of death to enhance their love and appreciation.
My answer: It is a warped individual who needs the fear of death to enhance his appreciation and love of another. I would assert that such a relationship is not healthy.
How does Kass believe this fear of death enhances love and appreciation?
Perhaps as follows: I was pretty sure I was going to die in 40 years of old age, but now I have been given a treatment which has extended my life indefinitely. Do I love my children less? How about my spouse? How about parents? How about granny? How about friends? Apparently the answer for Kass is yes, he would love and appreciate others less. For me the answer is no. Maybe Kass would only be motivated to be with friends and family more if he knew there was limited time to do so, otherwise he would blow them off. I think that the quality of relationships is relatively independent of lifespan.
Fourth, there is the peculiarly human beauty of character, virtue and moral excellence. Kass argues: if you cannot sacrifice your life then you are less virtuous and moral.
My answer: one who lives a lot longer could sacrifice multiple 120 year segments of life. Using Kass's point of view this person would be more moral and virtuous. My view is that there is no reduction in virtuousness or morality based on longer lifespan.
Kass's claims that these things will be diminished if we live longer are without evidence. If we reverse his assumptions and dial things back 60 years to when people had shorter lifespans, or if we go from the first world to the third world, do any of these arguments hold true? Is someone in North America or Japan less serious, aspiring, virtuous, moral or loving than someone with a shorter lifespan in Africa?
Bill McKibben, who wrote the book Enough, claims that human lives would no longer seem meaningful (11) in a world where such limitations (aging, constraints on physical and cognitive ability) could be overcome technologically.
A lot of McKibben and Kass's arguments seems to be that humanity should continue to battle death, but fail in this endeavor. They conclude that so long as we fail then our spirit will be good. I think this is crazy and wrong. Encouraging everyone to be a failure and a loser is wrong and immoral. It is still wrong even if McKibben and Kass have personal worldviews where they are mentally uncomfortable with less death and suffering. By their logic millions should die every year so that they might not have to re-examine whether some of their personal beliefs are wrong. Those who promote the continuation of death, suffering and failure are at best misguided, and at worst evil.
Accusations of religious motivation for those in favor of life extension
Some accuse those who are in favor of life extension and nanomedicine of having quasi-religious motivations. They label all those in favor of it to be Transhumanists, which they call a religion. (Transhumanism [sometimes abbreviated >H or H+] is an international intellectual and cultural movement supporting the use of new sciences and technologies to enhance human physical and cognitive abilities and ameliorate what it regards as harsh and unnecessary aspects of the human condition, such as disease and aging.)
I think this is a pointless argument. Let us assume the accusation was true, which I do not believe. Most of those who are opposed are usually staunchly religious. If being religious is not a flaw in them, then why is it a flaw for those with differing beliefs?
The range of new and prospective medical technology
Here is an additional summary of some controversial existing and potential medical technologies.
1.Genetic Manipulation
With the human genetic code now mapped, the race is on to find anti-aging genes.
Gene Therapy will probably be widely used to cure or treat muscular dystrophy. It seems likely to be more effective than steroids, and comes without the downsides. Millions will use it.
2. Stem Cells and Regeneration
While still a hot button issue, the potential of theraputic cloning and regenerative medicine using stem cells is enormous. Imagine growing a new heart from your own stem cells, creating a replacement organ without the dreaded problem of immune rejection from your body.
3. Small Biomechanical Devices and robotics
With smaller technology showing more and more promise, doctors are willing to take a look at MEMS and Nanotechnology for less invasive devices to monitor and repair aging cells and organs. In robotics, Bleex and other exoskeletons are working now and can be worn to boost the strength of the wearer. They will be used to help the elderly and for military purposes. Assuming Moore's law holds, there will be in about 30 years cheaply implantable petaflop computers.
4. Nanomedicine, which is discussed at the beginning of this article and at www.nanomedicine.com.
Let us look at a spectrum of treatments and enhancements to see how making something better does not transform it into something evil.
- hip replacement with metal
- hip replacement with artificial bone
- exoskeleton robotic
- internal pacemakers
- internal robotic components
- smaller internal robotic devices
- internal nanomedical devices
Why cut off better treatments? We should keep making things better
I will now pose a series of questions and situations, which I think illustrate the absurdity of not trying to make things better and how society has already made the choice that it is OK to augment human performance and to help save the dying and to improve the health of every person as much as possible.
In 20 years, Granny is wearing an exoskeleton and outperforming the ESPN4 world's strongest man without a suit. Why is this a problem? The world fastest man is slower than Granny when she is driving a car.
Super-granny with an exoskeleton. Maybe that's OK cause it is a tool on the outside. Hip replacement is under the skin. Well that's OK, it is just restoring some function. But if we combine some exoskeleton function with Hip replacement ? Is that banned ? Is it because granny is hiding enhancement under her skin ?
Oscar Pistorius, a South African who won bronze and gold in the 100 and 200 meter sprints respectively at the Athens Paralympics, and swept the events in last month's Visa Paralympic World Cup in Manchester, UK. Pistorius, 19, who is missing both legs below the knee, wears carbon fiber prosthetic devices. Those devices can be adjusted to enable a longer stride, an advantage in a running race. A likely development is for Pistorius to qualify for the able-bodied Olympics, a goal he is pursuing and one he might attain given his remarkable times (12). Advanced prosthetics are being attached to nerves, the brain and bone (13). They are being used to provide help to those who have lost limbs (like wounded U.S. soldiers) or who are deaf or blind.
Lasik eye surgery? Usually it is just restoring up to human level function. Well some get better than 20-20. what if they make it better. Everyone can have 20-5. 20-2. Have we crossed a line? Is it only OK to have inferior Lasik?
Some people say that we should not even think about curing ageing until people are assured of getting old in the first place. Then why give any treatment to anyone who is over the life expectancy of about 77 years of age? This is just giving an additional advantage to someone who has done better than the average expectation.
Should we research ways to help older stroke victims or heart disease patients? Maybe we should cut off that research and those treatments until we cure diseases and causes that hit earlier. This is tough luck for Granny and Granddad. They only thought they were lucky to live older. Maybe it is also tough for mom, dad and you and me too. By following that logic we should be concentrating money for diseases and problems at birth and childhood.
What seems like an argument for fairness to help those worse off first would result in trying to make everyone as sick as those who are the most unlucky. Not everything can or should be fair.
Stopping the development of better treatments hurts those who are more prone to die now. They are getting perpetual discrimination. The status quo is not always a safer or better choice.
Summarizing some "enhancement" questions society has already answered
- Making something better than what is replaced? Yes, Lasik eye surgery, advanced prosthetics.
- Making something better than a healthy human? Yes, Lasik eye surgery, cosmetic surgery, advanced prosthetics.
- Intergrate devices with human nerves and tissue? Yes, pacemakers, heart transplants, advanced prosthetics.
- Making something better than the current record holder? Yes, advanced prosthetics, Tiger Woods and other athletes with Lasik eye surgery.
- Fixing all-over, and not just a part of the body? Yes, Estrogen replacement, calcium supplements, many drugs in general.
- Research on helping older people live longer? Yes, heart and cancer research, diabetes research, Alzheimers and many other diseases.
- Inside or outside the skin? A factor? No, both are OK. Outside: high performance wheel chairs. Inside: hip replacement, organ transplants.
- Permanent part of the body? A Factor? Not a factor. There are many implanted stents, pacemakers and other devices.
- Gene therapy? Yes, there is no disagreement about using this for disease treatment. For other uses it is controversial.
Conclusion: Society will be able to successfully adapt to better health and lifespan increases
Past increases in lifespan and health have made for a richer and better society.
Those who oppose the development of better medicine when we could create it are in favor of continued suffering, deterioration, and unnecessary death. The arguments that society will suffer are without a firm basis. A false assumption is that society will not constructively adapt to improved health and longer lifespans.
We already have widespread internal and external devices that enhance our capabilities, both physical and mental. The cases that are made for the rejection of superior performance or finer grained modifications are inconsistent and harmful to individuals, individual rights, and the productivity of society.
(1) definition from nanomedicine.com
(2) Micro-Electro-Mechanical Systems (MEMS) is the integration of mechanical elements, sensors, actuators, and electronics on a common silicon substrate through microfabrication technology. memsnet.org
(3) link
(4) link
(5) link, link
(6) sens.org
(7) link
(8) link
(9) link
(10) link
(11) link
(12) link
(13) link
A longer list of the arguments and points in the argument are at wise-nano.org
A summary of a conference that discusses the ethical arguments for and against the right to enhancement technologies are at:
IEET Conference - Comprehensive Report - Part 1
IEET Conference - Comprehensive Report - Part 2
IEET Conference - Comprehensive Report - Part 3
Additional Links
Existence is Wonderful: The Fallacy of "Fix the World First"
Human Enhancement Technologies and Human Rights
Institute for Ethics and Emerging Technologies
|
Brian Wang is a long time futurist, who has been involved with nanotechnology associations since 1994. He is now a member of the Center for Responsible Nanotechnology (CRN) taskforce, and is moderating the technology sub-taskforce. He is also on the Nanoethics Group advisory board.
Wang has a degree in computer science and an MBA (from Canadian universities) and has worked in the information technology industry for 20 years. He created and ran his own professional services computer consulting company with offices in Canada and the United states and clients in the USA and Europe.
He won second place in the Honeywell University Futurist essay contest. He has been involved in nanotechnology as a Senior Associate of the Foresight Institute since 1997, and he helped write Foresight's 2003 relaunch plan.
Wang has a nanotech blog which we encourage you to visit at advancednano.blogspot.com
|
|
Interview with J. Donald Payne of Nanospectra Biosciences
|
Nanospectra Biosciences has an exclusive license to a new class of materials developed at Rice University, AuroShell™ microparticles (originally called nanoshells). While there are numerous therapeutic, diagnostic and industrial commercial opportunities, Nanospectra is focused on the development of a therapy broadly applicable to virtually all solid tumors, AuroLase™ Cancer Therapy.
AuroShell™ microparticles were developed in the laboratories of Naomi Halas, PhD, at Rice University in the 1990s. In collaboration with Jennifer West, PhD, of Rice University, a series of life science applications were envisioned which led to the formation of Nanospectra. Formal operations commenced in 2002 to commercialize applications using AuroShell™ microparticles.
Nanospectra is focused on the development of AuroLase™ Therapy to selectively destroy solid tumors. We currently intend to seek FDA approval to commence a human trial for the treatment of head and neck cancers in 2006. Other cancer applications will be developed after these initial trials.
NN: When you talk to non-scientists, how do you describe your AuroShell™ technology and AuroLase™ Therapy?
Our AuroLase Therapy is a particle-based thermal destruction of cancer, enabled by a new class of materials developed at Rice University which we call AuroShell particles. You can penetrate tissue with light in the near infrared wavelengths, but there previously were no biocompatible materials that absorb this wavelength. We construct particles small enough to be delivered to a tumor from the blood stream, but uniquely designed to absorb near infrared. Thus, when we shine a laser on a tumor area, our AuroShell particles will absorb this light. Anyone who has placed their hand on a metal surface in the summer sun knows that metals convert absorbed light to heat. In this same way, our particles in the tumor convert the laser light to heat to thermally destroy a tumor. And, unlike drugs and radiation, there is no immunity to heat – it can kill all cells.
While you can burn tissue in other ways, our particle-based approach allows the treatment of irregular shaped tumors, inoperable cancers and areas of regional disease. This is all enabled by the special properties of this new class of materials.
AuroShell particles can be uniquely tunable to absorb or scatter different wavelengths of light. Consisting of a thin gold metal shell surrounding a glass, or silica, core, this structure appears hollow when it interacts with light, giving it special optical properties and creating, in essence, a new biocompatible material for medical applications.
NN: What is your vision for the AuroShell™ microparticles and AuroLase™ Therapy?
We believe AuroLase Therapy will have broad applications in cancer. We will initially focus on head and neck cancer to fill the significant unmet medical need in this serious cancer, and then expand to other cancers after FDA approval. We hope to start our first human trial later in 2006. While cancer is our focus, our AuroShell particles have broad uses in other areas, which will be pursued as time and resources allow.
|
J. Donald Payne
Mr. Payne is the President and CEO of Nanospectra Biosciences, a Rice University spin-out developing medical therapeutic and diagnostic applications. As CEO, he has led NBI through preclinical development of its broad-based cancer therapy and plans to move this technology into clinical trials by the end of 2006. Mr. Payne has held progressively senior positions in the life science industry since 1992, holding executive positions with biopharmaceutical, tissue engineering and diagnostic companies. Prior to 1992, he held executive positions in the energy industry. He has an MBA from Rice University and a BBA from Texas A&M University.
Nanospectra Biosciences
8285 El Rio Street, Suite 150
Houston, Texas 77054
(713) 842-2720
Fax (713) 440-9349
|

|
Interview with Peter Searson, Director, Johns Hopkins Institute for NanoBioTechnology
|
NN: Please talk about the Institute for NanoBioTechnology; how it came to be; it's goals; and current status.
The Institute for NanoBioTechnology was launched on May 15, 2006. The Institute was conceived and created by a team of faculty from four divisions across the University: Peter Devreotes (Department of Cell Biology, School of Medicine), Michael Edidin (Department of Biology in the Krieger School of Arts and Sciences), Jon Links (Department of Environmental Health Sciences, in the Bloomberg School of Public Health), Marty Pomper (Department of Radiology, School of Medicine), Denis Wirtz (Department of Chemical and Biomolecular Engineering, Whiting School of Engineering), and myself (Department of Materials Science and Engineering, Whiting School of Engineering).
Over the last few years it has become recognized that nanotechnology has a critical role to play in health care and medicine. Hopkins is one of the few institutions with the faculty expertise, the research facilities, and the resources to undertake such as challenge. This was the motivation to create the Institute. The launch was the culmination of 18 months planning, discussions with faculty, and meetings with the university administration.
During the planning period we quickly realized that solving the complex scientific and engineering issues associated with the diagnosis and treatment of diseases and other medical conditions requires multidisciplinary teams. This provides a tremendous challenge in linking together faculty and students across the university. We believe that the key to solving this problem is to integrate research, education, corporate partnerships, and technology transfer across traditional departmental and divisional boundaries. Examples include, multidisciplinary graduate training programs, an undergraduate nano-bio minor, an industry affiliates program, and seed funding for research collaborations.
The goal is for Hopkins to become a leader in nano-biotechnology.
NN: What areas of expertise will the Institute be drawing on, and how do you see them collaborating?
The Institute will link faculty with expertise in molecular synthesis, device fabrication, surface science, molecular engineering, cell biology, diagnostics, therapeutics, and ultimately, clinical trials.
NN: How is the Institute being funded?
We have support from NASA to launch the Institute. We also have support for new graduate nano-biotechnology training programs from NSF and the Howard Hughes Medical Institute.
NN: Looking out ten years, what are your hopes regarding medical diagnostics and treatments stemming from our understanding of the nanoscale?
Very simply, we will develop new scientific tools and create new technologies for the diagnosis and treatment of diseases and medical conditions. We will develop new tools will allow us to develop a better understanding of how cells function, and misfunction, at the molecular level. Research will also focus on the development of new diagnostic and therapeutic strategies, for example, for the early detection and treatment of cancer.
|
Peter Searson
Searson is Director for the Johns Hopkins Institute for NanoBioTechnology. He is also a professor in Materials Science and Engineering in the Whiting School of Engineering and is Associate Director of the Materials Research Science and Engineering Center. He served as Chair of the Department of Materials Science and Engineering from 1997 - 2003. His research interests are in the synthesis and characterization of nanostructured materials and the applications for nanotechnology in biology and medicine, and he has more than 160 papers in scientific journals.
About the Johns Hopkins Institute for NanoBioTechnology
The Institute for Nanobiotechnology has been established at Hopkins to bring together expertise from the fields of nanotechnology, biotechnology, biology, medicine, and engineering to enable the creation of new knowledge and new technologies. In partnership with research facilities and universities throughout the country, the INBT will revolutionize health care and medicine by creating groundbreaking technologies based on nanotechnology.
The Institute's vision includes the development of innovative, interdisciplinary research programs focused in four interrelated thrust areas--therapeutics, diagnostics, cellular and molecular dynamics, and health and the environment. Interdisciplinary educational programs, public outreach programs, as well as industrial outreach programs at INBT are designed to foster the next generation of nanobiotechnology research and development.
|

|
Building The Winning Start-Up Team: Part 5 of 6
By Bo Varga
|
Introduction: Bo Varga has helped start-up & early stage high technology companies close funding, customers, and people, including recruiting senior technical and executive talent. Bo is currently recruiting team members for a nano separations start-up with major price-performance benefits in metals recovery for environmental, industrial, and mining applications. He is also consulting with a project for a media portal in China.
Article 1: Why Hire an External Recruiter? (Click here for a quote, and to here to buy.)
Article 2: Building The Winning Start-Up Team: Performance Requirements
Entrepreneurs, start-up teams, investors, and recruiters often intersect to match a startup with the experienced business management required for success.
Click here to read (for free) the rest of this report in full.
Article 3: The Recruiting Process
This article addresses the actual recruiting process an entrepreneur or team can use and the knowledge, background and reference checking to establish baseline trust in a new team member.
(Click here to buy.)
Article 4: Covered: Hiring, Motivating, Retaining Key Employees - the CEO example
(Click here to buy.)
Article 5: What investors and customers look for in start-up companies
Introduction:
Often nanotechnology companies are started by scientists or engineers with limited or no business experience. We have worked with people who do not know what a "bill of materials" or a "whole product" is. These entrepreneurs often have a standard set of weak spots in their management skills - especially a lack of investor focus or customer focus. Even companies with the strongest product/market potential and IP can fail to close required funding or fail to close or keep key customers, if the team does not include people who can "talk the talk and walk the walk" with investors and customers.
Article 5: What investors and customers look for in start-up companies
Objectives: Upon completion of this article, you will understand the key elements in the team that a professional investor or a major corporate customer needs to see in order to work with the start-up or early stage nano company. We include joint ventures, strategic alliances, and OEM relationships - as well as direct sales - in the customer category.
Elements in common are covered in section 1, elements specific to investors are covered in section 2, and elements specific to customers are covered in section 3.
1) Investors and Customers:
Investors & customers know that working with a start-up or early stage nano venture is risky. More to the point, whether working with a venture capital fund or a large corporate strategic ally, someone on the other side of the table has to stand up and be your "champion" and risk their own credibility, reputation, and possibly career path by buying into your venture. Often "gate keepers" have to pass your venture as suitable for an investment or vendor status. And "decision makers" have to agree to funding your company, purchasing your product, or making other commitments to your company to move you into the fast growth development path.
How can these key people be moved to engage with your nano venture in the first place? And how can you move them to a positive decision and positive action.
It all depends on your team, how they position and present themselves, what they say and do and how they say and do it. It is all about building trust and reducing perceived risk and managing expectations. And about using all your stakeholders as part of your virtual team.
Positioning and presentation are usually handled by PPT/PDF presentations, executive summaries, product data sheets, company brochures, company web sites and the like. We focus on quality presentations, clean layout and graphics, relevant information - different presentations for different targets from a master set, and staged presentations.
That is, initially the person or team on the other side of the table wants to explain their interests and how you can help them meet their business goals. Your positioning and presentations should be focused on the space where your needs and business goals intersect theirs - so much information is available on line and through relationship networks that there is no excuse for not understanding the general positioning and interests of the other team.
As you build your relationship your positioning and presentation needs to adapt to the specifics of the situation - you need to meet the needs to gatekeepers, identify and close with a champion, and move decision makers to your desired goals.
Your relationships and those established via your stakeholders can get you in the door and help convince gatekeepers, champions, and decision makers to work with you. After that your success depends on your actions and behavior.
Your stakeholders include anyone in your business network who will work on your behalf because they are engaged with your team, stand to benefit from the success of your business, or have prior knowledge & experience working with your team. Besides your founders, employees, and consultants, your stakeholders include your accountants, lawyers, board of advisors & directors, consultants in marketing, technical, & other areas, customers & vendors (including your landlord), as well as the prior business network of founders, employees, and contractors usually thought of as the team.
Introduction to investors or customers by a trusted source who references your company or team is the fastest way to start building a relationship. Ongoing support by a trusted source can help build relationships and work through problems, including miscommunications that occur in every relationship.
You can cast a much wider net to establish relationships using trusted sources by setting up a formal process that includes:
(i) identifying stakeholders who can help you with targeted investor or customer networks - best done by profiling the kinds of investor or customers you are targeting including specific sources so you can both identify those you know and learn about those you do not.
(ii) working a process to discover who will help you - you will need to know the nature of the relationship and how much time and energy the individual can and will commit on your behalf as a reference and to get and keep their commitment to help as needed - including participating at "get acquainted meetings," phone conferences, emails, etc.
(iii) aggressively following up on relationship leads provided by stakeholders - this is a balancing act - especially at the beginning of a relationship too many phone calls, emails, etc. can position you as a nuisance while too few indicates a lack of interest. In general we recommend and use staged communications, that is new information should be provided in each communication "we just got our second patent issued" to help move the target towards your goal.
(iv) keeping your active references in the loop as you build relationships with the people they referenced - copying them on key emails, calling them or meeting them regularly for coffee, sending "thank you" notes, etc.
Is there an easy way to do this? Ideally there is someone on your team who has worked marketing, sales, or business development and for whom the above process is part of his professional repertoire and for whom planning & implementation of this process is second nature.
While this process may seem like a great deal of work, in our experience it is not because a very few contacts will lead to the desired result, at least in Silicon Valley. For example with a recent start-up company we contacted 10 investors and 10 consultants we know who work investment networks. The 10 consultants referenced another 10 investors. We checked web sites, on-line biographies, and other publicly available information on the 20 target investors, narrowed the list to 7, and got strong investment interest from 5. The company actually pitched only 5 investors, 3 offered to lead and 2 to participate in any syndication.
We work as consultants, either directly with the founding team as temporary members or with the founder, entrepreneur, or CEO to help with positioning, presentations, and networking to targeted investors and customers.
We work on a retainer and transaction fee basis and are paid in stock before funding and post funding with a combination of cash and stock.
There are many individuals and teams in Silicon Valley that provide business development and marketing services if the company team does not have an experienced individual on board.
Of course we recommend the earliest possible hiring of a qualified CEO for funds raising and of a VP Marketing, VP Marketing & Sales, VP Business Development or similar.
However the start-up often has to gain some traction with investors or customers before a qualified individual is willing to risk his or her career, credibility, or reputation to join a new venture, so consultants are used to provide the required help.
Finally action and behavior are key to building and maintaining strong positive relationships.
We recommend a team focus to stay "on message" regarding the current company positioning and presentation, to "under commit and over deliver" rather than to "over commit and under deliver," to always address any concerns or problems from the other party directly and immediately, and to build trust with the other party and a reputation for professionalism and strong concern for the requirements and needs of the other party. "On time, on spec, and on budget" is the best bottom line summary for any business relationship.
2) Investors:
Investors understand that a start-up or early-stage company has more vision than reality, more flexibility than stability, and that the most important factor for success is the energy, focus, and perseverance of the team. That is, a strong team with a weak idea is more likely to succeed than a weak team with a strong idea. And investors also understand that the team is fighting the odds against success and therefore needs to be opportunistic, to rapidly shift gears when a major new opportunity is created, to use a business plan as a roadmap and not as a straight jacket. Sometimes teams do not understand this and stick to a business plan longer than useful or productive, so investors can act as a "reality check" at monthly Board of Directors meetings.
Investors are most concerned with the ability of the entrepreneur, founder, or inventor to be able to clearly plan what the team will do with the money and how they plan to repay the investor, usually by selling the company or taking it public. The team is focused on building a company, the investors on making an extraordinary return within a fixed time period - usually no more than 5 years. Investors see and invest in many deals, the team has only one company. The investors want to be as helpful as possible - including using their experience to prevent problems - the team often sees the investor as interfering, controlling, or trying to dominate the company without knowing the technology, market, or product details required for success.
Clearly there are built in tensions in the relationship between investors and the nano venture and the team needs to have on-board or as a stakeholder people who understand and can work with these tensions in a productive manner. That is, someone who talks investor.
Investors prefer not to replace the CEO or other team members but they prefer even less losing their money.
3) Customers:
Customers have a much more specific technology, market, product focused that is often more in tune with a founding team. However, they also understand the time and costs of delivering a "whole product," that is, commercializing a technology so that it delivers the whole package - documentation, service, support, upgrades, drivers, interfaces, etc. And they also understand the time, costs, & difficulties of scaling up to the volume required to gain the market share that will earn the profit that justifies the investment.
A start-up or early-stage company is risky to do business with. For example major corporations often require such a company to put into escrow the IP, technology, BOM, source code, etc. - all the elements required to produce the target product in case the small company goes out of business.
Start-up or early stage teams often do not understand product market dynamics - existing supply chains and value chains will react to new, breakthrough technology with defensive measures - from the large company's point of view the issue is what the product market dynamic is when the new technology or product is in mass production, not the situation today.
And start-ups are on a different time schedule - they need to show results fast - both to keep the team motivated and to keep attracting capital or market share or the other factors required for success. Fast for a major corporation may mean 5 to 10 years and for the nano venture 5 to 10 months. A middle ground must be found to make the relationship a success.
A scientist, engineer, or similar founder without this knowledge or experience will have difficulty convincing a champion or decision maker to proceed with the commitment, investment, and opportunity cost to partner with, buy from, or work with the nano venture.
Once again, we recommend recruiting people who understand and can work with established players. "A start-up cannot rely on another start-up for market penetration."
Negotiation of the joint venture or strategic alliance can be successfully concluded working with investment bankers, business development consultants, and marketing consultants and we have worked in these roles. At times it may make sense to enter into long-term relationships with consultants who have the required connections, experience, and knowledge and who only work part time for the nano venture.
This can both reduce fixed costs for the team and bring on-board people who are too expensive to hire as full time employees. The last and final article in this series will address the tradeoffs between full time employees, part time but long-term consultants, and short-term contractors.
Article 6, the final article in this series, "Building the Winning Nano Team" will help you identify gaps in team capabilities and understand how to fill those gaps through employees, consultants, boards of directors, and boards of advisors. The article will cover relevant aspects of the Bell Mason Diagnostics and the Stage Gate Business Development Process and how these can help to shape the nano venture's planning & implementation of team building.
Please address all comments and inquiries to: Bo Varga, bvarga@USnano.biz, skype siliconvalleynano.
© Copyright 2006 Bo Varga
|
Bo Varga is the Managing Director of Silicon Valley Nano Ventures.
Bo has 30 years business development and team building experience. His primary focus is to bring money to companies via angel, corporate, or VC investment, strategic alliances, development partnerships, or OEM sales. Bo has operations, sales, & marketing management experience in computer software & peripherals and in leading edge reconfigurable computing systems. He has worked with wireless, nanotechnology, reconfigurable computing, information technology, & ecommerce companies in team-building or business development roles.
He has helped executives, investors, and Boards of Directors for software, hardware, IS/IT, molecular engineering, & wireless companies by finding key team members and consultants for both technical & business positions.
His experience includes working as a strategic consultant to develop & implement marketing plans & presentations, with a specific focus on affiliate & event marketing to close business transactions. His focus since 2000 is on building global nanotechnology business networks via the nanoSIG & various nanotechnology conferences, forums, and symposiums. He is Chair of the NanoMaterials & Manufacturing Forum. Since 2001 he has organized over 60 nanotechnology events. His education includes a BA & MA from the University of Chicago and the MBA program in Accounting at UC Berkeley.
For more information on his work, see www.nanoSIG.org, www.USnano.biz.
He can be reached at bvarga@USnano.biz, or 650-747-9238 for more information.
|
Return to Top
Nanotechnology and Nanomedicine Artwork can be viewed at these locations:
The Nanomedicine Gallery
Nanotechnology Now Art Gallery
Gobblebot: Artificial White Blood Cell
Tim Fonseca
(click to enlarge)
|
Generic Nanobots
Tim Fonseca
(click to enlarge)
|
Regarding the artwork: artwork in these galleries comes from the fertile imaginations of both artists and scientists. Each piece represents the "idea" of a particular device. Should it eventually be build, the actual device may bear little resemblance to the artist's concept - it will however, likely perform as expected.
Quotes
An ultrasensitive DNA and protein detector, expected to be widely available later this year, could save lives by detecting genetic and infectious diseases early, before they turn deadly or spread. Its relatively low cost and simplicity will make diagnostic tests that today can be done only in specialized labs available at local hospitals. Furthermore, because it's extremely sensitive, it could detect signs of disease invisible to current tools.
The device, which has been developed by Nanosphere, Northbrook, IL, based on research by Chad Mirkin, professor of chemistry at Northwestern University, is already being in used in several research labs and is awaiting Food and Drug Administration approval before it enters general use. link
"A revolution has begun in science, engineering and technology, based on the ability to characterize, manipulate and organize matter systematically at the nanometer scale. Nanotechnology is beginning to provide the ability to work at this level -- to control nanoscale structures, using them as building blocks to construct larger material components, systems and architectures to form supramolecular structures with fundamentally new molecular organization. Within these larger scale assemblies, the control and construction of substructures and components remains at the nanometer scale. Nanotechnology offers opportunities for the creation and utilization of materials, devices and systems by building from the level of atoms and molecules. The key resultant opportunity is the exploitation of novel and improved properties that emerge at the nanoscale. Nanotechnology is expected to play an important role in scientific disciplines such as physics, material science, biology, medicine, engineering and computer simulation." National Institutes of Health (NIH). From "Nanoscience and Nanotechnology in Biology and Medicine" Link
There are signs that we are in the midst of an explosion of new health-related technologies. Genomics, proteomics, stem cells, structured-based drug design, photodynamic therapy, combinatory chemistry, and intercellular signaling, provide tremendous insight and new directions for medical research. When nanotechnology and nanoscience are used along with these technologies, the likelihood of significant medical advances and breakthroughs is unprecedented.
Nanotechnology Tools will allow researchers to visualize, measure and manipulate items at the nanoscale such as cells, bacteria and viruses, and to detect single molecules. Among other things, this will provide new information about biological processes for understanding cellular function including real time measurements of single molecules in living cells and the development of the collateral chemistry and instrumentation.
Nanomaterials will solve unique biological and medical challenges with synthetic and hybrid nanostructures that can be functionalized to carry out highly focused tasks. Examples include sensing and repairing biological lesions and damages just as biological nanostructures (e.g. white-blood cells and wound-healing molecules); detecting and treating diseases after measuring various parameters such as electrical changes from biological molecules; drug delivery systems that can pass through natural defense mechanisms including the blood-brain barrier; and longer lasting and better performing materials for bone replacements, prostheses, and implants. As life contains a collection of molecular materials and biological structures in the nanoscale, nanostructured materials are ideally sized to complement nature. —Neil Gordon, President of the Canadian NanoBusiness Alliance
Like primitive engineers faced with advanced technology, medicine must "catch up" with the technology level of the human body before it can become really effective. What is the technology level? Since the human body is basically an extremely complex system of interacting molecules (i.e., a molecular machine), the technology required to truly understand and repair the body is molecular machine technology -- nanotechnology. A natural consequence of [our achieving] this level of technology will be the ability to analyze and repair the human body as completely and effectively as we can repair any conventional machine today. — Brian Wowk, "Cell Repair Technology" link
We envision biocompatible surgical nanorobots that can find and eliminate isolated cancerous cells, remove microvascular obstructions and recondition vascular endothelial cells, perform 'noninvasive' tissue and organ transplants, conduct molecular repairs on traumatized extracellular and intracellular structures, and even exchange new whole chromosomes for old ones inside individual living human cells. —Robert A. Freitas, Jr. link
Nanomedicine is here now. Drug delivery from inhalers to tablet formulation and polymer dissolution and phase change studies have been part of Nanomedicine for years. At Veeco we have seen strong growth in both atomic force microscope and interferometer sales for drug discovery, development and production QC over the last two years. Nanomedicine will continue to grow in these and many less traditional applications over the next 10 years. &$0151;Clark Taylor, Veeco Instruments
We're very optimistic that nanotechnology can markedly improve cancer therapy. —James Baker, Director, the Michigan Nanotechnology Institute for Medicine and the Biological Sciences.
The only medicines that ever seem to "fix" anything are molecular medicines like antibiotics and cancer drugs, and macroscopic, mechanical therapies like bone setting and surgery. But surgery is incredibly crude cutting big holes through a person to get at something inside -- usually something really small. There's a lot of room to do better, with smaller holes and more versatile instruments, and with nanosurgery as the end result. Also, working up from the molecular level, we may start to see polyfunctional, shape-changing molecules which can serve multiple duties inside the body. Why cut at all, when you can send in the molecular mechanics and fix the problem at its root? —Wil McCarthy link
The revolutionary promise of molecular nanotechnology (MNT) has become a part of society's expectations for the future. This technology will provide nanomedicine breakthroughs that could cure cancer and extend lifespace, bring abundance without environmental harm and provide clean sources of energy. These ideas are part of the vision that launched the field of nanotechnology. —K. Eric Drexler link
So while I acclaim this bill (1) as a fantastic first step, there's a lot more that can and should be done. We are still spending less than $1 billion per year on nano, which puts it in the company of a lot of minor, unimportant government programs. Nano is more significant than that, and we should consider beginning a truly ambitious program. Every day we delay is a day that we spend hundreds of millions of dollars buying oil from countries that hate us. Every day we delay is a day that we let thousands of people around the world die who could be saved by nanomedicine.
—James Von Ehr, from Bolder nano R&D needed to reduce foreign-oil dependence 21st Century Nanotechnology Research and Development Act (PDF)
Most of my work is oriented toward the longer term, but if I had to guess, the (nanomedicine) applications nearest to commercialization are probably the fullerene-related and dendrimer-related drugs. The nanoshells are making their way toward commercialization, but the fullerenes and dendrimers are probably closest in terms of somebody making money from a product. —Robert A. Freitas, Jr.
Far from being a dream, nanotechnology will materially impact many of our economies largest markets during the next 10 years, and will be a common thread in many of the emerging businesses during this time. While medicine will lag other industries due to the multi-year average time for new medical technologies to emerge from clinical trials, it may be the most profoundly impacted within the next two decades. —Dr. Andrew Mutz, Managing Director Codesta
As an emerging science in its infancy, nanotechnology promises the nano-scale manufacture of materials and machines made to atomic specifications. It is a field at the junction of chemistry, physics, biology, computer science and engineering. In the context of this paper, nanotechnology will be referred to as a manufacturing as well as a computational science, since many of tomorrow's manufactured items exist today only as models and simulations. The impact of nanotechnology on our way of life is widely believed to reach profound and hitherto unimagined levels in the coming decades. Proposed changes include clean abundant energy, pollution-free and inexpensive production of superior defect-free materials, complete environmental restoration and cleanup, safe and affordable space travel and colonization, and quantum leaps in medicine leading to perfect health and immortality. As a result of these advances, we anticipate the obsolescence of nearly all of today's industrial and economic processes by the first half of the new century, leading to global and radical changes in life style, finance, law, and politics. —Behfar Bastani, Dennis Fernandez link
In the next 30 years, we may experience more technological advances -- from nanoscale manufacturing, medicine, education and leisure -- than the amazing progress realized during all of the 20th century. Research at the nano level will teach us how to build new materials and tiny structures by assembling atoms or molecules with high precision instead of the more conventional approach of sculpting parts from pre-existing materials. —Mihail C. Roco, Senior Advisor, NSF and Chair, U.S. National Science and Technology Council's Subcommittee on Nanoscale Science, Engineering and Technology. link
News
"Nanomedicine" News: May 01, 2006 - May 31, 2006
Fourth Funding Round of the Texas Emerging Technology Fund
businesswire May 31, 2006 The Houston Technology Center (HTC) announced today that the Gulf Coast Regional Center of Innovation and Commercialization (GCRCIC) is now accepting applications for the fourth round of funding for the Texas Emerging Technology Fund (TETF) - including Life Science applications.
Beneq acquires business of ABR Innova
kauppalehti.fi May 31, 2006 Beneq Holding Oy has entered into a business transfer agreement with ABR Innova Oy whereby Beneq has acquired all the nHalo business from ABR Innova Oy.
Nanoforce Announces Signing Definitive Agreement to Merge
primezone May 31, 2006 Nanoforce, Inc. (Pink Sheets:NNFC), a developer of nano-materials, new refining processes, and equipment for use in alternative and existing energy sector technologies, announced the signing of a definitive agreement to merge between Transglobal Oil, Corp. (Pink Sheets:TGOC), a Delaware corporation, and Energy Farms, Inc., a wholly owned subsidiary of Nanoforce incorporated in the State of New Mexico.
pSivida directors take up entitlements under rights issue
wabusinessnews.com.au May 30, 2006 Directors of Perth headquartered global bio-nanotech company pSivida Ltd have confirmed that they will be taking up rights to subscribe for pSivida shares under their respective individual eligible entitlements to raise approximately $29 million.
pSivida also announced the Regulatory Agency in the U.K. has granted approval to proceed with the first human study of its BrachySil product in pancreatic cancer through a phase IIa clinical trial.
Stock Trading Alert
blackenterprise.com May 30, 2006 Investors may want to have a look at Industrial Nanotech, Inc. (PINKSHEETS: INTK), a company that specializes in nanotechnology innovation and product development. " The revenue potential for our products in the food & beverage industry is substantial and we are preparing to achieve the proper registrations necessary to enter this industry segment internationally," (said) Francesca Crolley, Industrial Nanotech V.P. of Operations & Marketing.
ARM Working with UK Universities On Nanotechnology
ARM May 30, 2006 ARM is amongst a group of companies working with researchers from five UK universities on nanotechnology that will power the technology of the future.
State funds sci-fi project
frontiersman.com May 30, 2006 Nanotechnology sounds like something straight out of Star Trek. But the best things are supposed to come in small packages, and thanks to nanotechnology - the manipulation of tiny elements - we enjoy a host of new developments, including razor-thin cell phones, pocket-size music players and smaller computers.
All that high-tech and sci-fi stuff might be coming to the Mat-Su Valley, thanks to a $100,000 grant from the state capital budget. Wasilla Rep. Vic Kohring added the funds to the budget for the study and development of microscopic technology.
Technology Park Created in Knoxville-Oak Ridge
prnewswire May 30, 2006 Business leaders and officials in the Knoxville-Oak Ridge Innovation Valley announced plans today to build the nation's first technology park located at a national laboratory.
Higgins Announces $2 Million for NanoDynamics
NanoDynamics May 30, 2006 Today, Congressman Brian Higgins (NY-27) announced that he secured $2,000,000 in federal funding for NanoDynamics Inc, a leading company in the rapidly growing field of nanotechnology, located along Buffalo's Outer Harbor. The funding will be used for the design and construction of a fuel cell system operating through the use of methane gas, a by-product of many water treatment facilities.
Building an engine for Springfield's rebirth
boston.com May 29, 2006 Robert Forrant: While Springfield stumbled into economic depression, political leaders and economic development experts denied that a day of reckoning would come. But, years of downtown store closings, rising office vacancies, dubious fiscal management, corruption on a grand scale, and the drain of state revenues from the city to subsidize the Big Dig plunged the city into near bankruptcy.
Policymakers and economic development professionals must focus their efforts on the generation of employment opportunities in technology fields related to biotechnology, medical instrumentation, environmental measuring and testing devices, and nanotechnology.
Baby Breaker Birth Announcements
fool.com May 27, 2006 Tim Beyers: Next up is nanotech. The small science has been attracting plenty of investor attention lately, and I'm not just talking about Motley Fool Rule Breakers pick Harris & Harris (Nasdaq: TINY), either. Witness OVP Venture Partners.
Canada lags in nanotechnology
canada.com May 26, 2006 Canada risks losing top researchers in nanotechnology if it does not promote the industry, a panel of experts agreed yesterday. A lack of a government policy to promote nanotech and disinterest by large firms are keeping Canada far behind other developed countries, members from universities, businesses and government agencies argued during Nanotechnology Day at Concordia University. "And that's a shame, because there are so many great researchers here," said Neil Gordon, president of the Canadian Nanobusiness Alliance.
SA's R&D spend begins to turn the corner
engineeringnews.co.za May 26, 2006 The department is also making progress with its nanotechnology ambitions, and South Africa's Minister of Science and Technology, Mosibudi Mangena announced that it has published the Nanotechnology Strategy. Nanotechnology, and its building block, nanoscience, promise to produce smaller, cheaper, lighter and faster devices with greater functionality that use fewer raw materials and consume less energy. “Nanotechnology will drive the business revolution into the 21st century. We are determined to ensure South Africa joins the international competitiveness race that Nanotech expertise promises to provide.”
Harris & Harris Seeks Some Sun and Surf
fool.com May 26, 2006 Jack Uldrich: The venture capital firm, which specializes in investing in nanotechnology-related start-ups, announced last month that it was the lead investor in a $7.5 million Series B funding round for Innovalight. ... given solar energy's impressive growth rate, Harris & Harris' move looks like a prudent investment in a technology that is poised to move into the mainstream in a big way.
The firm's other recent investment appears considerably riskier. Just last week, Harris & Harris disclosed that it had invested a modest amount in D-Wave Systems
Big Business Gets Small
fool.com May 26, 2006 Jack Uldrich: This past week, I had the opportunity to attend the Fifth Annual NanoBusiness Alliance Conference out in New York City, and I was impressed with how some of the Fortune 100 companies viewed nanotechnology as a critical component of their overall growth strategy. For these companies, nanotech is not simply a long-term research-and-development project that might lead to some promising breakthroughs in five to 10 years, but rather a practical field of science that's being applied to improve existing products today.
NAIT publicly unveils details of its $750 million campus expansion plan
cnw.ca May 25, 2006 (Including) Centre for Electrical Technologies, opening in August 2011, will be housed in the former eight-storey business tower, and expand enrollment by 35% in Biomedical Engineering, Electrical Engineering, Electrician and Electronic Service Technician programs. This building will also provide space for emerging technologies such as Robotics, Industrial Animation, and Nanotechnology.
OMSI gets grant for nanotechnology exhibit
oregonlive.com May 25, 2006 OMSI has received a two-year, $712,000 grant to develop exhibits, education programs and public forums that will explore benefits and concerns about nanotechnology -- a fast-growing engineering field that manipulates atoms and molecules to fabricate new materials and tiny devices.
UF's big into nanotechnology
gainesville.com May 25, 2006 At UF, nano is all the rage. Currently, UF researchers have some $40 million to $50 million in nano-related contracts, and that number is likely to go up very soon, according to UF officials.
The university is looking to become a major player in the field, breaking ground June 15 on a $35.2 million nanosystems facility that will bring together chemists, physicists, medical doctors and engineers under one roof to work on nano projects.
A VC sees green
canada.com May 25, 2006 Menlo Park-based Kleiner Perkins plans to set aside $100 million of its latest $600-million fund for technologies that help provide cleaner energy, transportation, air and water. That's on top of more than $50 million Kleiner Perkins had already invested in seven greentech ventures. Also known as clean technology, the field includes technologies related to water purification, air quality, nanotechnology, alternative fuels, manufacturing, recycling and renewable energy.
Veridium Executes Agreement
businesswire May 25, 2006 Veridium Corporation (OTC Bulletin Board: VRDM) today announced its execution of agreements with GreenShift Corporation (OTC Bulletin Board: GSHF), Veridium's majority shareholder, to acquire the stock of GreenWorks Corporation and GS CleanTech Ventures, Inc.
Dow Opens Research Center at Cambridge
advancedimagingpro.com May 25, 2006 Dow Corning Corporation, a member of the Centre for Advanced Photonics and Electronics (CAPE) consortium, announced that Cambridge University Electrical Engineering Division has completed and equipped a new research facility for the development of emerging technologies across a variety of markets, including optoelectronics, nanoelectronics and displays.
NanoDynamics To Recieve $2 Million in Federal Funding
fuelcellsworks.com May 25, 2006 Washington, DC-Today, U.S. Representative Brian Higgins (D-Buffalo) announced that he secured $2,000,000 in federal funding for NanoDynamics Inc, a leading company in the rapidly growing field of nanotechnology, located along Buffalo's Outer Harbor. The funding will be used for the design and construct of a fuel cell system operating through the use of methane gas, a by-product of many water treatment facilities.
ISE-CCM Nanotechnology Index
dbusinessnews.com May 24, 2006 Cronus Capital Markets and the International Securities Exchange jointly developed the index, which has options currently trading on the ISE (ISE:TNY).
Wise nanoeducation investment or nano-pork?
nanodot May 24, 2006 Christine Peterson: Given the increasing protest from U.S. voters over the growing problem of “pork” spending by Congress, it behooves us in the nanotech field to develop criteria for nanotech projects.
Xerox Nanotechnology in Canadian Industry Award
Canadian NanoBusiness Alliance May 24, 2006 For most outstanding technological innovation and corporate leadership in the application of nanotechnology in a product or process
Virginia Tech materials researchers to improve military armor
eurekalert May 24, 2006 Virginia Tech has been selected by the Army Research Laboratory to establish a Materials Center of Excellence. The center will develop polymer-based materials to protect personnel and equipment against weapons attack. The center will also offer graduate student and postdoctoral scholar mentorship and undergraduate research programs. The ARL award provides $500,000 per year, potentially renewable for nine years, totaling approximately $4 million, Long said. "It is a prestigious award for Virginia Tech. These funds will have a tremendous impact on advancing nanotechnology research on campus.
NVE Awarded DoD Development Contract for Anti-Tamper Sensor
prnewswire May 24, 2006 NVE Corporation (Nasdaq: NVEC) announced today that it was recently awarded a contract by the U.S. Army to develop a magnetic anti-tamper sensor.
N.J. officials look to N.Y. for insight on building nano-tech infrastructure
ACBJ May 24, 2006 New Jersey wants to get into the nanotechnology game and is taking stock of where the nation's strengths are. Albany, N.Y., is clearly on the radar of state officials.
UD receives $2.1M grant for tooling
daytondailynews.com May 23, 2006 The University of Dayton was approved for $2.1 million in state grants Monday for a project facilitating the use of new polymer nanocomposite materials in tools for manufacturing.
Tree-Hugging Capitalists
forbes.com May 23, 2006 Erika Brown: Clad in hiking pants and Birkenstock sandals (with socks), Doerr's co-emcee Bill Joy looked more like a tree-hugger than a calculating VC. But he's clearly been running the numbers: "There is a big wave coming as Moore’s Law meets the biotech and nanotech revolutions. Opportunities abound in new materials, new pathways, new organisms, new forms of magic."
Investor Alert for Tuesday, May 23rd, 2006
marketwire May 23, 2006 eLocity's stocks to watch for today are -- Industrial Nanotech, Inc. ... Investors may want to have a look at Industrial Nanotech, Inc. (PINKSHEETS: INTK), a global nanoscience solutions and research leader.
M'sia To Build Nanotechnology Centre This Year
bernama.com.my May 22, 2006 A proposal to build a National Nanotechnology Centre is now at the final stages, said Science, Technology and Innovation minister Datuk Seri Dr Jamaludin Jarjis.
OVP Venture Partners VII holds first closing on $207m
altassets.com May 22, 2006 OVP will continue to focus on the communications and network infrastructure, digital biology, semiconductors and electronics, software and nanotechnology sectors. Including the new fund, OVP now has over $700m under management.
Nanosphere aims for diagnostics market
smalltimes May 22, 2006 Nanosphere just raised $57 million from investors who believe that the company's use of nanotechnology in the ultra-sensitive pursuit of molecular materials can offer a big improvement over the current generation of diagnostic tools on the market.
Starpharma: SPHRY vs SPHRF
NanoNovus May 22, 2006 Jack Uldrich: Last week, I received an inquiry from a reader asking about the difference between two different stock listings for Starpharma.
SMI announces Air Force Phase I SBIR
SMI May 22, 2006
Materials Science & Engineering logs banner year
University of Washington May 22, 2006 UW Engineering's Department of Materials Science & Engineering, the oldest of the engineering disciplines on campus and a contributor to such high-profile projects as NASA's Space Shuttle program, is reporting a banner year for research funding, having garnered nearly $18 million since last spring. (Including at least 6 nanoscale projects)
The American Competitiveness Initiative
whitehouse.gov May 21, 2006 Today, President Bush Discussed His American Competitiveness Initiative (ACI) - A Strategy To Keep America The Most Innovative And Competitive Economy In The World. The ACI will encourage more aggressive investment by businesses in research and development, increase Federal support for vital basic research, and improve math and science education for America's students.
President Bush Is Doubling The Federal Commitment To The Most Critical Basic Research Programs In Physical Sciences Over The Next Ten Years. This increased funding will encourage scientists to explore promising areas such as nanotechnology, supercomputing, and alternative energy sources.
Luna Innovations Still Sees IPO Prices of $11-$13/Share
streetinsider.com May 21, 2006 In an amended S-1 filing with the SEC, Luna Innovations (NASDAQ: LUNA) indicated it still sees an IPO price of $11-$13 per share on 4 million shares. The company has applied to list their common stock on the Nasdaq National Market under the symbol "LUNA."
$1 Million Grant Goes to San Jose State
ascribe May 21, 2006 The U.S. Department of Defense has awarded a second major grant to enhance San Jose State University's Materials Characterization and Metrology Center, which provides students with hands-on experience in nanoscale materials research, a field that could revolutionize the manufacturing process worldwide. With the new grant, the number of nanotech students at SJSU will double to nearly 20, and more equipment will be purchased.
New Jersey business briefs
northjersey.com May 21, 2006 The state's Commission on Science and Technology said Friday it will give $500,000 to support the creation of NJ NANO @ RU, a nanotechnology facility at the Institute for Advanced Materials and Devices at Rutgers University in New Brunswick.
Hi-tech park gets a re-think
vnagency.com.vn May 21, 2006 Although the Sai Gon Hi-tech Park (SHTP) has achieved significant success and registered investment of US$712 million in three years of operations, it requires an additional VND2.23 trillion (US$140 million) to develop infrastructure to lure more investors, according to park officials.
"As part of the first stage, the park earmarked about VND180 billion for two research and development centres focused on semi-conductors and nanotechnology. It is also building a training centre and a precision mechanics research facility."
Europeans to make construction kit of tailored nanomotor components
nanodot May 21, 2006 Christine Peterson: As described — briefly, as one would expect — in the May 2006 Nanotech Briefs, the EU is funding a nanomotor construction kit project.
QuNano AB Announces Closing of Series A Financing Round
QuNano May 21, 2006 QuNano AB of Lund, Sweden, today announced the closing of its SEK 45 million (USD 6.1 million) Series A investment round. Founded in September 2005, QuNano is a technology spin-out from the Nanometer
Structure Consortium (“nmC”) at Lund University and is focused on commercialisation in the
fields of nanoelectronics and photonics of the heterostructured nanowire technology
research of Dr. Lars Samuelson, Professor of Solid State Physics, and his world-class team
at Lund University.
Biophan Sells One Million Shares of Common Stock to SBI
businesswire May 19, 2006 Biophan Technologies, Inc. (OTCBB: BIPH; FWB: BTN), a developer of next-generation medical technology, announced today that it has exercised a call requiring SBI Brightline XI, LLC to purchase 1 million shares of Biophan Common Stock at a price of $2 per share, as part of its financing agreement with SBI.
German ITN Nanovation eyes 50 mln euro IPO
reuters.com May 19, 2006 German nanotechnology firm ITN Nanovation plans to float in June, selling shares worth up to around 50 million euros ($64 million), sources familiar with the deal said. The firm, which makes coatings for power plants for German firms, plans to make an initial public offering (IPO) to finance expansion abroad.
£52m boost for Higher Education innovation
egovmonitor.com May 19, 2006 Targeting the early signs of disease, boosting the number of entrepreneurs, and getting UK creative industries into China are some of the award winning projects sharing in £52 million for higher education institutions, Trade and Industry Secretary Alistair Darling announced today.
The eleven competitive winning bids include: University of Leeds: White Rose Health Innovation Partnership. To develop new methods, not yet tried in the UK, to stimulate innovation in healthcare using the experience of medical technologies in the US. The next ten years will see radical treatments and technologies covering nanotechnology, biomedical materials and sensor technology that will focus on targeting prevention diagnosis.
OVP Venture Partners Closes Largest Venture Capital Fund
businesswire May 19, 2006 Early-stage venture capital firm OVP Venture Partners (OVP) has held a first closing on OVP Venture Partners VII (OVP VII) at $207 million, above its target of $200 million. It is the largest fund the partnership has ever raised, with a final closing planned later this year. The firm now has over $700M under management.
Altair Stock Component in Clean Edge
marketwire May 19, 2006 Altair Nanotechnologies Inc. (NASDAQ: ALTI) today announced that its common stock was selected for inclusion in the NASDAQ® Clean Edge® U.S. Index.
Singular ID and Veneto Nanotech incorporate Singular ID Italia Srl
Singular ID May 18, 2006 Singular ID Pte Ltd, the provider of integrated brand security products based on advanced micro and nanotechnology solutions, announced today that it has incorporated its Italian subsidiary Singular ID Italia Srl with an investment from Veneto Nanotech SCPA.
Singular ID was the winning team in the Nanochallenge International Business Plan competition, held during November 2005. The prize of €200,000 cash and €100,000 in-kind services will fund the initial operations of Singular ID Italia, enabling it to rapidly build its business from its location in northern Italy. At incorporation, Veneto Nanotech holds 7.5% of the quotas of the subsidiary, the
balance being allotted to Singular ID. (PDF)
RPI, UAlbany get state grants for tech-transfer projects
ACBJ May 17, 2006 The funding was among the $5.3 million in state grants for 13 projects announced Tuesday by the New York State Office of Science, Technology and Academic Research's Technology Transfer Incentive Program. The industries and colleges promised to commit a total of $6.9 million as their shares of the projects.
BioNanomatrix Increases Seed Funding and NCI Grant
prnewswire May 17, 2006 BioNanomatrix LLC, an emerging company developing a breakthrough nanoscale whole genome imaging and
analytic platform, today announced receipt of a two-year grant from the National Cancer Institute and new seed financing from two venture investors - New York-based 21 Ventures LLC and Ben Franklin Technology Partners of Southeastern Pennsylvania.
FEI Investment Conference Webcast
FEI May 17, 2006 FEI Company (NASDAQ: FEIC) announces the following Webcast: FEI Company Robins Emerging Opportunities Investment Conference Webcast.
JMAR Technologies Receives Nasdaq Notification
businesswire May 17, 2006 MAR Technologies, Inc. (NASDAQ:JMAR) announced that on May 12, 2006, it received a letter from the Nasdaq Stock Market confirming that for the last 30 consecutive business days prior to May 12, 2006, the bid price of the Company's common stock has closed below the minimum $1.00 per share requirement for continued listing set forth in Marketplace Rule 4310(c)(4).
Q&A: Axiom’s Scott Livingston
redherring May 17, 2006 Scott Livingston, managing director of Axiom Capital Management, sounded chipper Wednesday, and for good reason. Nanotechnology, Mr. Livingston’s specialty, is looking prettier to many investors these days.
Nano investments shift to applications
EETimes May 17, 2006 "We know that getting from idea to commercialization of actual nanometer-sized circuits takes about ten years, so we are picking our targets judiciously," Vida Ilderem, vice president of technology at Motorola Laboratories, told the NanoBusiness 2006 conference.
Nanosphere Raises $57M
redherring.com May 16, 2006 Nanosphere said Tuesday it has raised $57 million in venture capital that will enable it to commercialize its first product later this year. Nanosphere plans to use the money to launch the Verigene System, which provides more sensitive genetic testing at a lower cost than conventional machines. The system, which enables doctors to check for a patient’s predisposition for certain diseases, consists of two instruments and use gold nanoparticles to identify genetic materials.
AFMNet and industry partners invest $15 million
newswire.ca May 16, 2006 At its Second Annual Scientific Conference held in Calgary this month, the Advanced Foods and Materials Network (AFMNet) announced the funding of 20 innovative food and bio-materials related research projects. (Including:) University of Guelph - Foods: Structure, Growth and Nanotechnology Applications; and Protein and Peptide Self-Assembly: Food-Derived Materials and Interactions with Nanostructured Surfaces.
ARM and Freescale work with UK universities on nanotechnology
electronicsweekly.com May 16, 2006 ARM, Freescale and National Semiconductor are among a group of companies working with researchers from five UK universities on a project to investigate the feasibility of new types of semiconductor transistor which are significantly smaller than those used in current ICs.
Nanotech Investment Outlook
robtv.com May 16, 2006 Video interview with Neil Gordon, President, Canadian NanoBusiness Alliance
Nanotechnology is the "future"
ACBJ May 16, 2006 State Comptroller Alan Hevesi told a group of companies and investors interested in nanotechnology that the state is committed to investing to help them grow. He said that $420 million of the $250 million in the New York State Retirement Fund was committed to high-tech companies -- an intentional misstatement -- then joked, "I'm not good with numbers."
Next Floor - Men's Fashion, Sporting Goods and the Ionosphere ...
prnewswire May 16, 2006 ZiLOG(R), Inc., (Nasdaq: ZILG) a leading provider of integrated microcontrollers (MCUs) and universal remote control solutions, today announced that it is providing a range of its award-winning 8-bit microcontroller solutions and development tools to support a team of 30 innovative students from the University of British Columbia (UBC) in their bid to win the 2006 NASA Beam Power Challenge space elevator design competition.
Harris & Harris Group Invests in D-Wave Systems
businesswire May 16, 2006 Harris & Harris Group, Inc., announced today that it led a $14 million second round financing in D-Wave Systems, Inc. of Burnaby, British Columbia, Canada.
Washington U. researchers work to bring ideas to market
stltoday.com May 16, 2006 In a couple of weeks a team of Washington University's star researchers will begin a novel experiment, with broad implications for the region's economy as well as for human health.
Drs. Samuel Wickline and Gregory Lanza are moving their lab into the first building of Cortex, a budding biotech business corridor near midtown, to launch the Consortium for Translational Research in Advanced Imaging and Nanotechnology, or C-TRAIN.
Foolish Forecast: Applied Materials
fool.com May 15, 2006 On May 4, Applied Materials announced its intention to acquire nanotech company Applied Films(Nasdaq: AFCO), which will expand the company's business in the realms of flat-panel displays and flexible electronics, as well as in solar cells and energy-efficient glass. In other news, in March, Applied Materials announced that it is cutting short last year's $4 billion stock buyback program just over halfway through its run, and replacing it with a new, $5 billion program that will run over the next three years.
Informz moves software development to Albany Nanotech
ACBJ May 15, 2006 As part of the move, Informz and the college have formed a partnership to jointly develop nanotechnology-related information technology programs focused on research and development, and work force training.
FEI Company Announces Proposed Notes Offering
FEI May 15, 2006 FEI Company (Nasdaq: FEIC) today announced that it intends to offer, subject to market conditions and other factors, $100 million aggregate principal amount of convertible subordinated notes due 2013.
Magazine Preview: Nanotech: Payoff Time
redherring May 15, 2006 When Kevin Matthews took the reins of Oxonica in 2001, the then two-year-old nanotechnology company was gunning for a highly competitive electronic display market but still had no products. It was also running on empty, with only 12 weeks of cash left in the bank. Four years later, the Oxford spin-off has over $20 million in cash from big investors and is turning out products like a fuel-efficient diesel additive and performance-enhancing sunscreen compounds.
Eau Claire nanotech center receives $1.5M federal grant
ACBJ May 15, 2006 The Chippewa Valley Technical College in Eau Claire will receive $1.5 million from the U.S. Department of Commerce for construction of a $5.5 million nanotechnology incubator.
Local uni leads way to gizmos of future
eveningtimes.co.uk May 15, 2006 Glasgow University is heading a £5.3million project to develop a new generation of gizmos so small it could create vital medical equipment that can be placed in the body and act as a tiny hospital lab.
Lawmakers devour huge, oil-fed surplus
adn.com May 14, 2006 When legislators arrived in Juneau in January facing a billion-dollar budget surplus, they vowed to be careful and try to salt away half the money for the future.
In the closing days of the legislative session last week, it seemed caution was being thrown to the wind. Over the final weekend, the House Finance Committee added $275 million in scores of local-interest projects to create a record-breaking capital budget. Among the last-minute projects: nanotechnology development in the Mat-Su Borough ...
Stepping Up the Development of Science and Technology
korea-np.co.jp May 13, 2006 SPA Chairman Choe Thae Bok (The People's Korea, North Korea): In the field of hardcore basic technology it is urgent to build a nationwide information network and develop programming technology rapidly and thus turn our country into a power in software development. Efforts should be channeled into building a nano material industry and producing good breeds by dint of bio-engineering. It is important to lay more solid foundations for the development of space technology and oceanography and direct efforts to the basic scientific researches so as to intensively solve the theoretical and methodological problems pending an urgent solution for the development of the latest science and technology.
Group will use $5 million to lure high-paying industries
idahostatesman.com May 12, 2006 The Valley Initiative for Prosperity will be a five-year program to market the Treasure Valley to relocation experts used by companies interested in moving to new cities. Industries to be targeted include computers and electronics, transportation, warehousing, software, telecommunications, health care, biotechnology, nanotechnology and equipment manufacturing.
IBM Goes Back to School
fool.com May 12, 2006 Jack Uldrich: 'Tis the season for college acceptance letters, and we all know that the schools a student applies to can say a lot about his or her plans. And whether or not the student is accepted is often a fair predictor of that student's long-term success.
Much the same can be said for the association that corporations often make with academic institutions -- such as the one IBM (NYSE: IBM) recently formed with Rensselaer Polytechnic Institute (RPI) to build a $100 million supercomputer.
FEI Company Authorizes $20 Million Stock Repurchase Program
FEI May 12, 2006 FEI Company (Nasdaq: FEIC) today announced that its Board of Directors has authorized the repurchase of up to $20 million of the Company's common stock. Share repurchases under this program may be made through open market and privately negotiated transactions, at times and in such amounts as management deems appropriate.
Nanotechnology initiative gets financial boost
knoxnews.com May 11, 2006 Technology 2020 is receiving $100,000 from the U.S. Department of Commerce's Economic Development Administration to support the Innovation Valley Nano Alliance, with $50,000 in matching funds coming through Jobs-Now!, a regional economic development initiative. Tech 2020 pitched in the remaining $50,000 in matching funds required for the federal grant.
Raymor Closes Financing
marketwire May 11, 2006 Raymor Industries Inc. (TSX VENTURE:RAR), a developer and producer of single-walled carbon nanotubes, nanomaterials and advanced materials is pleased to announce the following: The company has closed a private placement for 8,333,333 units, representing proceeds of $10,000,000 and the TSX Venture Exchange has accepted this private placement, previously announced on April 27, 2006.
Which ETF is ready to move?
tradingmarkets.com May 11, 2006 Deron Wagner: One such ETF that many traders are not aware of, yet has been acting great, is the PowerShares Lux Nanotech Fund (PXN). This ETF, which is comprised of various stocks associated with nanotechnology, has been in a steady uptrend since its launch in October 2005.
Investments Tips for Aggressive Traders!
marketwire May 11, 2006 Nanoforce, Inc. (OTC: NNFC) may be of interest to speculative investors this morning. Yesterday after the stock markets closed, the company, a developer of nano-materials, new refining processes and equipment for use in alternative and existing energy sector technologies, issued a press release announcing the incorporation of a new, wholly owned, subsidiary, Energy Farms, Inc. in New Mexico.
Uni professor a $1.5million bionics man
illawarramercury.com.au May 11, 2006 Professor Gordon Wallace yesterday received the $1.5 million five-year fellowship to conduct further nanobionics research at the University of Wollongong.
Europe Still In Danger of Missing the Boat - Yawn....
TNTlog May 11, 2006 We always like to read these reports as if the word 'nano' had been removed, and see if they lose anything by it. In the case of most of the conclusions, the answer is no. Europe is historically terrible at linking academic R&D with commercial success, Asia does dominate micro electronics and Japanese car manufacturers do dominate hybrid motor technology.
Nanogen Receives $25 Million Equity Financing Commitment
prnewswire May 10, 2006 Nanogen, Inc. (Nasdaq: NGEN), developer of advanced diagnostic products, announced today that it
has received a commitment for up to $25 million in common stock equity financing from Azimuth Opportunity Ltd. Subject to the terms and conditions of a purchase agreement between Nanogen and Azimuth Opportunity, over the next eighteen months, Nanogen may sell registered shares of its common stock, at its discretion, to Azimuth Opportunity at a small discount from market price.
PChem Receives Investment from Ben Franklin Technology Partners
PChem Associates May 10, 2006
$100 million supercomputing center to RPI
ACBJ May 10, 2006 Called the Computational Center for Nanotechnology Innovations, the new center will focus on reducing the time and costs associated with designing and manufacturing nanoscale materials, devices and systems, officials announced Wednesday.
A Tale of Two Nanotech Companies
NanoNovus May 10, 2006 Jack Uldrich: In the past two days, two nanotech companies – Headwaters and Altair – have reported earnings. I encourage investors to pay close attention to these figures.
Stadium mania is producing illusion of forward movement
startribune.com May 10, 2006 Jack Uldrich: ... I was flying to Albany was to give a talk on the emerging field of nanotechnology, and to congratulate the region on the success of its vibrant public-private "NYLovesNano" -- a five-year public-private partnership that has already added $1.1 billion in new revenues and 1,500 high-tech jobs to the region.
The region, which soared from No. 131 to No. 66 in the Milken Institute's latest rankings of regional economic competitiveness in the past year, is not content to rest on its laurels. This January, Gov. George Pataki announced the creation of a $435 million initiative to create a new Institute for Nano-Electronics Discovery and Exploration.
My point is this: While Minnesota is poised to squander hundreds of millions on questionable stadiums, other regions are investing in the future.
Research earns top reward
icnetwork.co.uk May 09, 2006 You wouldn't recognize scientist Sharon Louch as she goes about her work in face mask and protective clothing. Sharon ... has gained recognition with an award for her cutting-edge research. Sharon received £500 for her thesis on magnetic nanoparticles and says she's delighted by the award.
Wall Street News Alert: Traders' Alert for Tuesday!
marketwire May 09, 2006 Wall Street News Alert's "stocks to watch" this morning are: Nanoforce, Inc. (OTC: NNFC) should be a "stock to watch" for aggressive investors and day traders this morning! The company, a developer of nano-materials, new refining processes and equipment for use in new and existing energy sector technologies, issued a press release announcing that it has entered into an agreement to acquire Vancouver based Clemco Industries Inc.
Industrial Nanotech Retains New York Securities Firm
primezone May 09, 2006 Industrial Nanotech (Pink Sheets:INTK), a company that specializes in nanotechnology innovation and product development, announced the retention of Great Eastern Securities, Inc. to advise the Company on matters relating to the capital markets such as project financing, analyst coverage and stock exchange listing.
Brazil's INMETRO Selects FEI for New Nano-Materials Center
FEI May 09, 2006 FEI Company (Nasdaq: FEIC) announced that Brazil's National Institute of Metrology, Standardization and Industrial Quality (INMETRO) has selected FEI to supply a suite of tools that enable nanoscale materials research. The selected systems include FEI's ultra-high resolution Titan(TM) S/TEM, a Tecnai(TM) TEM and a Nova NanoLab 600 DualBeam(TM) tool. These systems will be used by the institute and other Brazilian institutions at INMETRO's new nano-materials center in Rio de Janeiro.
Bruker BioSciences, Optics union will deliver molecular analysis systems
manufacturing.net May 08, 2006 Bruker BioSciences Corp. (BRKR) plans to acquire all the stock of molecular spectroscopy company Bruker Optics Inc. for $135 million, payable 59% in cash and 41% in BRKR stock.
Nanoforce Acquires Clemco Industries
primezone May 08, 2006 Nanoforce, Inc. (Pink Sheets:NNFC), a developer of nano-materials, new refining processes and equipment for use in new and existing energy sector technologies, announced that the Company has entered into an agreement to acquire Vancouver based Clemco Industries Inc.
South Lagging on Nanotechnology Research and Industry
localtechwire.com May 08, 2006 The South is lagging other regions of the nation in the opportunity to capitalize on the rapidly growing nanotechnology industry, according to the Southern Growth Polices Board. The 14 states that make up the South represent some 20 percent of all nanotech research technology and features four of the top 25 nanotechnology-related research institutions, the RTP-based group said in a new report.
NanoMaterials Technology and Sydney University Awarded Grant
prnewswire May 08, 2006 Singapore-based NanoMaterials Technology Pte Ltd ("NMT"), together with the University of Sydney, has
been awarded an Australian Research Council Linkage Grant, with a total project funding of US$333,000. This is for research into the high gravity precipitation of nanoparticles for pulmonary drug delivery.
Rubincon Ventures and API Electronics Merger Agreement
marketwire May 08, 2006 Rubincon Ventures, Inc. (OTC BB: RBCV), and API Electronics Group Corp. (OTC BB: AEGCF), today announced the signing of a definitive agreement to merge in an all-stock transaction that was previously announced on March 27th, 2006.
Senate subcommittee looks to nano for economic boost
smalltimes May 08, 2006 The U.S. Senate Commerce Committee's Subcommittee on Trade, Tourism and Economic Development heard representatives from universities, business and government describe nanotechnology as the frontier of a new global competition at a hearing on Capitol Hill Thursday.
"Nanotechnology has tremendous potential to improve the quality of life for our citizens, create high paying jobs, and increase U.S. global competitiveness," said Sen. Gordon Smith (R-OR), who presided over the hearing. "Unfortunately, the government has not made the economic development aspect of nanotechnology much of a priority. We're going to try to do that."
NSF: Redesign science curriculum
eschoolnews.com May 08, 2006 To strengthen the nation's global competitiveness, the National Science Foundation is calling on education leaders to redesign their K-12 science curricula to incorporate the latest discoveries in biotechnology, nanotechnology, and other fields, and to allow more time for student inquiry. To lead these efforts, the agency has committed $1.8 million to the College Board to revise the curriculum for Advanced Placement science courses.
Nanotechnology in $32 billion worth of products
Lux Research May 08, 2006 Lux Research releases The Nanotech Report, 4th Edition, the indispensable reference guide to nanotechnology
NanoVenture Competition Held in Hawaii
genengnews May 08, 2006 The American Society for Mechanical Engineers' NanoVenture Competition 2006 is looking for the next great nanotechnology startup. The competition is geared towards startup companies looking for their first round of venture funding.
NanoNovus: May 8
NanoNovus May 08, 2006 Jack Uldrich: This article from the Miami Herald provides a good overview of nanotechnology’s ability (and limitations) to detect cancer at an early stage. On the positive side, nanotechnology is leading to the development and creation of new diagnostic technologies that can detect unique “biomarkers”–specific proteins that show up when a cancer is present.
On the down side, these biomarkers are not always either sensitive or specific to a single disease. This suggests that even if new diagnostic technologies are developed, they might not do that much good.
Australia Backs Nanotech Research for ''Real-World'' Applications
businesswire May 08, 2006 Australia, which is globally recognized for its Government-backed research facilities that consistently spin-out commercially focused innovation, will this year present some of its best R&D at NSTI Nanotech 2006 (May 7-11, 2006).
Nanotechnology research could provide cures
thestar.co.za May 06, 2006 Nanotechnology - the "technology of the small" - is set to blaze new scientific trails in South Africa, and one of the most innovative research fields involves using gold in the fight against HIV/Aids. The Department of Science and Technology recently launched a national nanotechnology strategy for South Africa, which will include a R450-million investment over the next three years.
Scott Livingston speaker at NYS Investors conference
eisinc.com May 05, 2006 Rene M. LeRoux, Executive Director of the NYS Investors-Nanotechnology Conference is pleased to announce that Scott Livingston will be a special guest speaker at the conference.
Lumera Receives Purchase Order from Harvard
businesswire May 05, 2006 Lumera Corporation (NASDAQ:LMRA), an emerging leader in the field of nanotechnology, announced today that it had received a purchase order from Harvard Medical School (HMS) for its beta ProteomicProcessor(TM) Biosensor instrument.
Applied Materials to Expand Nanomanufacturing Technology Solutions
businesswire May 05, 2006 Applied Materials, Inc. (Nasdaq:AMAT) and Applied Films Corporation (Nasdaq:AFCO) today announced that they have signed a definitive agreement for Applied Materials to acquire Applied Films. Applied Films is a leading supplier of thin film deposition equipment used in manufacturing flat panel displays (FPDs), solar cells, flexible electronics and energy-efficient glass. This acquisition will complement Applied Materials' thin film nanomanufacturing technology(TM) capabilities and provide Applied Materials with an opportunity to expand into growing new markets.
Politicos suggest more Fed spending on nanotech
news.com May 05, 2006 U.S. senators on Thursday said they're excited about nanotechnology's potential and are eager to throw more tax dollars into speeding the burgeoning materials from the laboratory to the marketplace.
"Unfortunately the federal government has not made the economic development aspect of nanotechnology much of a priority," Sen. Gordon Smith, an Oregon Republican, said at a hearing on commercialization of nanotechnology.
SMI announces completion of MDA Phase II award
Structured Materials Industries May 05, 2006
Veritek Technologies Inc. Proposed Qualifying Transaction
newswire.ca May 04, 2006 Veritek Technologies Inc. (the "Corporation") is pleased to announce the details outlining its proposed Qualifying Transaction with Omni-Lite E-FORM Technologies Inc. The Corporation has entered into a letter of intent dated April 11, 2006, for the acquisition of all the issued and outstanding shares (the "E-FORM Shares") in the capital of Omni-Lite E-FORM Technologies Inc. ("E-FORM").
E-FORM is developing a series of technologies to provide nanostructured materials to customers in the Aerospace, Military, Resource Development, Sports and Recreation and Medical Industries.
Murdock to Testify Before Senate
businesswire May 04, 2006 The NanoBusiness Alliance, the world's leading nanotechnology trade association, today announced that executive director Sean Murdock will testify today at a Senate Economic Subcommittee hearing on "Promoting Economic Development Opportunities Through Nano Commercialization."
NanoNovus: May 4 (FEI)
NanoNovus May 04, 2006 Jack Uldrich: FEI, one of the 15 companies I recommended that nanotech investors hold in their portfolio, is up over 16 percent today on news of very solid first quarter earnings.
HP and Trinity in €1.8m
siliconrepublic.com May 03, 2006 Science Foundation Ireland (SFI) has awarded a €1.8m contract to the Trinity College-based Centre for Research on Adaptive Nanostructures and Nanodevices (CRANN), which will work with tech giant HP to develop flexible electronic devices for the consumer electronics market.
NanoNovus: May 3 (IBM & NanoInk)
NanoNovus May 03, 2006 Jack Uldrich: The EE Times has an informative article on IBM’s latest nanotechnology advance. The advance is a significantly faster method for “writing” atoms and molecules onto substrates.
TECH 2006 Meets in Liverpool
oswegocountybusiness.com May 03, 2006 Following up on a major theme - nanotechnology - from 2004, the morning keynote address was given by Keith Blakely, CEO of Nanodynamics. “You are going to see nanotechnology change the way you live,” said Blakely before cautioning, “it's not hype, but that doesn't mean there is value in every nanotech company.”
pSivida Limited: Rights Issue to Fund Trials
businesswire May 02, 2006 Global bio-nanotech company pSivida Limited (NASDAQ:PSDV)(ASX:PSD)(Xetra:PSI) today announced details of a Non-Renounceable Rights Issue offering one new ordinary share for every eight shares held at May 22 ("the Record Date") at an issue price of AU$0.60 per share. Capital raised from this Rights Issue will primarily fund the phase III clinical trials of Medidur(TM) for the treatment of Diabetic Macular Edema (DME), and phase IIa clinical trials of our lead BioSilicon(TM) product, BrachySil(TM) which is being developed for the treatment of inoperable pancreatic cancer.
Raymor Prepares for Production of SWNT and US Listing
Raymor Industries May 02, 2006 Raymor Announces Year-End Results: 2005 Prepares Raymor for Numerous Accreditations, Production of Single-Walled Carbon Nanotubes, and Application for US Listing
Smart Imaging Technologies Secures Venture Funding
Smart Imaging Technologies May 02, 2006
3M sees capital spending at $1.5B to $1.8B by 2008
marketwatch May 02, 2006 Chief Executive George Buckley said that his focus will be on building 3M's technology base. He said 3M will look for more disruptive technologies - inventions that make more than an incremental advance in a market - and "just out of the garage" technology. Nanotechnology will be a key area, he said.
Educators, lawmakers hopeful about lottery-funded program
myrtlebeachonline.com May 01, 2006 Some of the proceeds from South Carolina's lottery have paid for eight endowed chairs at the state's research universities. The research areas of the approved endowed chair positions have focused on nanotechnology, health sciences, fuel-cell development and safety.
Cooper's funding requests
tennessean.com May 01, 2006 The following is the list of special budget requests submitted by Rep. Jim Cooper, D-Nashville. Advanced Carbon Nanotechnology Program at Vanderbilt University, $6 million: Would complete a four-year $14.5 million research project aimed at developing new chemical and biological weapon sensors, armor for soldiers and vehicles, and battery technologies.
Nanoeconomist is advocate for change
ACBJ May 01, 2006 Citing Lux Research statistics, Cupoli said nanotechnology-enabled product revenue will grow from $13 billion in 2004 to $2.6 trillion by 2014. At that time, the United States will need 2 million nanotech-savvy workers--400,000 scientists and 1.6 million highly skilled engineers, technicians, business leaders and, yes, economists.
The Capital Region has already seen the benefits of that, he said, noting that $8 billion has been invested and 2,500 jobs have been created in the 18-county Tech Valley region since 2002. Albany NanoTech's partnerships with companies such as IBM, Infineon and Sony will provide possibilities for students who want to land high-paying jobs in the area.
Solar Energy Developer Innovalight Raises $7.5 Million
Innovalight May 01, 2006 Innovalight, Inc., a privately-held firm focused on developing low-cost, nanotechnology-based printed solar cells, today announced that the company has raised an additional $7.5 million in private equity financing.
This Series B financing led by Harris & Harris Group, Inc., includes investment from existing investors Apax Partners, ARCH Venture Partners, Sevin Rosen Funds and Triton Ventures. Innovalight plans to employ this additional capital to accelerate the development of ultra-low cost, lightweight solar cells using a proprietary silicon ink-based technology. (PDF)
Nanotech center gets $10 million-plus pledge
ACBJ May 01, 2006 Georgia Tech's highly anticipated nanotechnology center has received an "eight-figure" pledge, the university says. The donation is contingent on Gov. Sonny Perdue's approval of state funding for the project, said spokesman Jim Fetig. That's expected to happen in May.
Harris & Harris Group Invests in Innovalight
businesswire May 01, 2006 Harris & Harris Group, Inc., announced today that it led a $7.5 million Series B round of financing of privately held Innovalight, Inc., of Santa Clara, California.
SCS Capital to Help Fund Nanopharm Project
blackenterprise.com May 01, 2006 Nanopharm Technologies (M) Sdn Bhd and its Russian partner, LLC Polymorph, have roped in venture capital firm SCS Capital Sdn Bhd to help fund its RM100 million pharmaceutical project.
.02%, Exponential Growth & Nano-enabled Solar Cells
NanoNovus May 01, 2006 Jack Uldrich: In my book, I begin by discussing the extraordinary power of exponential growth. To make my point I wrote the following: “Human tendency is to assume linearity. That is, most people assume progress will proceed in a prescribed, organized, and straightforward fashion. This line of thinking is best exposed with a short quiz...
From Our Molecular Future: How Nanotechnology, Robotics, Genetics, and Artificial Intelligence Will Transform Our World, by Douglas Mulhall:
-
What happens to the monetary system when everyone is able to satisfy his own basic material needs at very low cost?
-
How would we use cash when digital manufacturing makes it impossible to differentiate a counterfeit bill or coin from the real thing?
-
What happens to fiscal policy when digital information, moving at light speed, is the major commodity?
-
How fast will monetary cycles move compared to, say, the ten- or twenty-year cycles of the late twentieth century, when products and patents go out of date in a matter of months instead of years?
-
What happens when we don't have to worry about trade or social services for our basic needs, because most of what we need is provided locally with digital manufacturing, and the biggest trade is in information?
-
How do we control the excesses of the ultrarich, the overabundance of the molecular assembler economy, and the challenge to intellectual property laws created by intelligent, inventive machines?
-
What happens if half of all jobs are made redundant every decade?
-
What happens to the War on Drugs when there's no import, export, or transport of contraband because drugs can be manufactured in a desktop machine using pirated software downloaded from the Internet?
-
What happens to democratic controls when individuals can get as rich as small governments in a year or so?
-
What's the relevance of insurance if many things are replaceable at very low capital cost, but liabilities from software are potentially unlimited?
-
How should organized labor react when molecular assemblers and intelligent robots eliminate most manufacturing jobs?
-
What is the nature of work going to be?
-
What happens to land prices when an individual can build a tropical farm under a bubble in North Dakota, and get there from New York in an hour?
-
What happens when everyone can go everywhere, whenever they want, and work from wherever they want?
Return to Top
Useful Links
Glossary for NanoBiotechnology
Nanotechnology and Life Extension By Chris Phoenix
Our first report on nanomedicine, Sept. 2004
Our "Best of 2003" Award for Promising Discoveries
Adriano Cavalcanti on Medical Nanorobotics Feasibility
The Harrow Technology Report
Center for Responsible Nanotechnology
Return to Top
IN THE NEXT ISSUE
Issue #37 will cover Military Applications. It will land in your mailbox July 5th, 2006.
Infamous Quotes:
"This 'telephone' has too many shortcomings to be seriously considered as a means of communication. The device is inherently of no value to us." Western Union internal memo, 1876
"Heavier-than-air flying machines are impossible." - Physicist and mathematician Lord Kelvin, President of the British Royal Society, 1895
"Everything that can be invented has been invented." - Charles H. Duell, Director of U.S. Patent Office, 1899
"There is no likelihood man can ever tap the power of the atom." - Robert Milikan, Nobel Laureate in Physics, 1923
"Theoretically, television may be feasible, but I consider it an impossibility-a development which we should waste little time dreaming about." - Lee de Forest, inventor of the cathode ray tube, 1926
"I think there is a world market for maybe five computers." IBM's Thomas Watson, 1943
"Landing and moving around on the moon offer so many serious problems for human beings that it may take science another 200 years to lick them." - Science Digest, August 1948
"Computers in the future may weigh no more than 1.5 tons." Popular Mechanics, 1949
"There is no reason anyone would want a computer in their home." Ken Olsen, Digital Equipment Corp, 1977
And the lesson is? It's a tough game to call.
Need advice? Check out NanoStrategies
© Copyright 1999-2006 7thWave, Inc. All rights reserved. More information on copyright, and disclaimers regarding the content of this newsletter can be found here
|