Home > Press > Weird, water-oozing material could help quench thirst: Nanorods' behavior first theorized 20 years ago, but not seen until now
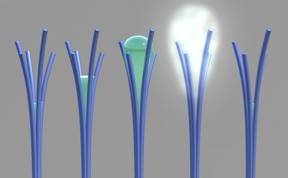 |
| PNNL researchers saw for the first time a phenomenon that was theorized more than 20 years ago. Shown here is a PNNL illustration of the phenomenon, "solvent cavitation under solvo-phobic confinement," which PNNL researchers saw occur with carbon-rich nanorods they mistakenly created. PNNL's viewing of the phenomenon involved liquid spontaneously evaporating after being confined within tiny spaces in between touching nanorods. Image from S. Nune et al, Nature Nanotechnology, 2016.
CREDIT: Pacific Northwest National Laboratory |
Abstract:
After their nanorods were accidentally created when an experiment didn't go as planned, the researchers gave the microscopic, unplanned spawns of science a closer look.
Weird, water-oozing material could help quench thirst: Nanorods' behavior first theorized 20 years ago, but not seen until now
Richland, WA | Posted on June 13th, 2016Chemist Satish Nune was inspecting the solid, carbon-rich nanorods with a vapor analysis instrument when he noticed the nanorods mysteriously lost weight as humidity increased. Thinking the instrument had malfunctioned, Nune and his colleagues moved on to another tool, a high-powered microscope.
They jumped as they saw an unknown fluid unexpectedly appear between bunches of the tiny sticks and ooze out. Video recorded under the microscope is shaky at the beginning, as they quickly moved the view finder to capture the surprising event again.
The team at the Department of Energy's Pacific Northwest National Laboratory would go on to view the same phenomenon more than a dozen times. Immediately after expelling the fluid, the nanorods' weight decreased by about half, causing the researchers to scratch their heads even harder.
A paper published today in Nature Nanotechnology describes the physical processes behind this spectacle, which turned out to be the first experimental viewing of a phenomenon theorized 20-some years ago. The discovery could lead to a large range of real-world applications, including low-energy water harvesting and purification for the developing world, and fabric that automatically pulls sweat away from the body and releases it as a vapor.
VIDEO
"Our unusual material behaves a bit like a sponge; it wrings itself out halfway before it's fully saturated with water," explained PNNL post-doctoral research associate David Lao, who manufactured the material.
"Now that we've gotten over the initial shock of this unforeseen behavior, we're imagining the many ways it could be harnessed to improve the quality of our lives," said PNNL engineer David Heldebrant, one of the paper's two corresponding authors.
"But before we can put these nanorods to good use, we need to be able to control and perfect their size and shape," added Nune, the paper's other corresponding author.
Expectations v. reality
Ordinarily, materials take on more water as the humidity around them increases. But these carbon-rich nanorods -- which the researchers mistakenly created while trying to fabricate magnetic nanowires - suddenly expelled a large amount of water as the relative humidity inside the specimen holder reached anywhere between 50 and 80 percent.
Water expulsion can clearly be seen in the microscope video. Water is visible as a gray, cloudy haze -- and only emerges from where nanorods intersect. When the team went on to raise the humidity further, the nanorods' weight also increased, indicating they were taking on water again. It was also reversible, with water being ejected and later absorbed as humidity was gradually lowered back down.
The team was further intrigued. They couldn't think of any other material that takes on water at a low humidity and spontaneously releases it at a high humidity. So they dug through the canons of scientific literature to find an explanation.
Old theory, new evidence
They found a 2012 paper in the Journal of Physical Chemistry B that explained how, in certain situations where liquid is confined in a teeny-tiny space (roughly 1.5 nanometers wide), the liquid can spontaneously evaporate. And the authors of a 2013 paper in the (Journal of Chemical Physics described how water can condense into the confines of close hydrophobic materials, which do not play well with water, and quickly turn into vapor due to attractive forces between the surfaces of the two materials facing each other. The 2013 paper gave this phenomenon a very long, technical name: "solvent cavitation under solvo-phobic confinement."
These papers also noted the process was theorized as early as the 1990s by scientists examining crystallized proteins. Back then, scientists noticed they only saw water vapor surrounding hydrophobic sections of protein, while liquid water would surround other areas. The researchers proposed that there was some sort of process that enabled the water caught between hydrophobic protein sections to suddenly vaporize.
Armed with this knowledge, the PNNL team hypothesized water was condensing and forming a bridge between the nanorods, through a process known as capillary condensation. Next, they believe water between rods forms a curved cavity whose surface tension pulls the adjacent rods closer together. When two intersecting nanorods reach about 1.5 nanometers apart, the team reasoned, the water caught between them could be forced to quickly evaporate.
Putting it to good use
Though understanding the nanorods' unexpected behavior is a triumph in itself, the PNNL team also foresees a future where this phenomenon could also improve quality of life. They see their discovery as a potential humanitarian lifesaver, describing it as "a paradigm shift in water purification and separation," in their paper.
Theoretically, large quantities of the water-spitting nanomaterial could repeatedly take on and then eject collected water when a certain humidity level is reached. Such a system could be used in remote deserts, where it would collect water from the air and harvest it for human consumption.
Another vision is to create a membrane that takes on and later expels water as humidity changes. The membrane could be used in jacket fabrics and enable more comfortable outdoor adventures by removing sweat from inside a jacket and emitting it outside as a vapor.
To make these applications possible, the team is exploring ways to make more of its nanorods spray water. The team estimates only around 10 to 20 percent of the material spits water right now. The plan is to scale up production of the current material, creating more than a few grams of the material at a time. They will do further analysis to ensure the phenomenon is still present when larger amounts are present. They are also conducting a more detailed examination of the material's physical and chemical properties and determining if other materials that have similar properties. The team is also intrigued by the idea other nanomaterials could potentially be developed to collect other liquids, such as methanol.
###
This research is funded by PNNL's internal Materials Synthesis and Simulation Across Scales Initiative. The project also used the expertise of staff and several advanced instruments -- environmental transmission and scanning electron microscopes, an X-ray photoelectron spectrometer and a Mössbauer spectrometer -- at EMSL, the Environmental Molecular Sciences Laboratory a DOE Office of Science User Facility at PNNL.
####
About Pacific Northwest National Laboratory
Interdisciplinary teams at Pacific Northwest National Laboratory address many of America's most pressing issues in energy, the environment and national security through advances in basic and applied science. Founded in 1965, PNNL employs 4,400 staff and has an annual budget of nearly $1 billion. It is managed by Battelle for the U.S. Department of Energy's Office of Science. As the single largest supporter of basic research in the physical sciences in the United States, the Office of Science is working to address some of the most pressing challenges of our time. For more information on PNNL, visit the PNNL News Center, or follow PNNL on Facebook, Google+, LinkedIn and Twitter.
EMSL, the Environmental Molecular Sciences Laboratory, is a DOE Office of Science User Facility. Located at Pacific Northwest National Laboratory in Richland, Wash., EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. Its integrated computational and experimental resources enable researchers to realize important scientific insights and create new technologies. Follow EMSL on Facebook, LinkedIn and Twitter.
For more information, please click here
Contacts:
Franny White
509-375-6904
Copyright © Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Textiles/Clothing
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() Polymer fibers with graphene nanotubes make it possible to heat hard-to-reach, complex-shaped items February 11th, 2022
Polymer fibers with graphene nanotubes make it possible to heat hard-to-reach, complex-shaped items February 11th, 2022
![]() Flexible material shows potential for use in fabrics to heat, cool July 3rd, 2020
Flexible material shows potential for use in fabrics to heat, cool July 3rd, 2020
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








