Home > Press > Platinum nanocrystals boost catalytic activity for fuel oxidation, hydrogen production
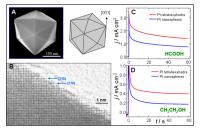 |
| (A) Low-magnification SEM image of a platinum tetrahexahedral nanocrystal and its geometrical model. (B) High-resolution transmission electron microscopy image recorded from a platinum tetrahexahedral nanocrystal to reveal surface atomic steps in the areas made of (210) and (310) sub-facets. Credit: Zhong Lin Wang |
Abstract:
A research team composed of electrochemists and materials scientists from two continents has produced a new form of the industrially-important metal platinum: 24-facet nanocrystals whose catalytic activity per unit area can be as much as four times higher than existing commercial platinum catalysts.
Platinum nanocrystals boost catalytic activity for fuel oxidation, hydrogen production
Atlanta, GA | Posted on May 3rd, 2007The new platinum nanocrystals, whose "tetrahexahedral" structure had not previously been reported in the metal, could improve the efficiency of chemical processes such as those used to catalyze fuel oxidation and produce hydrogen for fuel cells.
"If we are going to have a hydrogen economy, we will need better catalysts," said Zhong Lin Wang, a Regents Professor in the School of Materials Science and Engineering at the Georgia Institute of Technology. "This new shape for platinum catalyst nanoparticles greatly improves their activity. This work also demonstrates a new method for producing metallic nanocrystals with high-energy surfaces."
The new nanocrystals, produced electrochemically from platinum nanospheres on a carbon substrate, remain stable at high temperatures. Their sizes can be controlled by varying the number of cycles of "square wave" electrical potential applied to them.
"This electrochemical technique is vital to producing such tetrahexahedral platinum nanocrystals," said Shi-Gang Sun, an Eminent Professor in the College of Chemistry and Chemical Engineering at the Xiamen University in China. "The technique used to produce the new platinum nanostructures may also have applications to other catalytic metals."
The research was supported by the Natural Science Foundation of China, Special Funds for Major State Basic Research Project of China and the U.S. National Science Foundation. Details will be reported in the May 4 issue of the journal Science.
Platinum plays a vital role as a catalyst for many important reactions, used in industrial chemical processing, in motor vehicle catalytic converters that reduce exhaust pollution, in fuel cells and in sensors. Commercially available platinum nanocrystals - which exist as cubes, tetrahedra and octahedra - have what are termed "low-index" facets, characterized by the numbers {100} or {111}. Because of their higher catalytic activity, "high-index" surfaces would be preferable - but until now, platinum nanocrystals with such surfaces have never been synthesized - and therefore have not been available for industrial use.
The nanocrystals produced by the U.S.-Chinese team have high energy surfaces that include numerous "dangling bonds" and "atomic steps" that facilitate chemical reactions. These structures, characterized by {210}, {730} or {520} facets, remain stable at high temperatures - up to 800 degrees Celsius in testing done so far. That stability will allow them to be recycled and re-used in catalytic reactions, Wang said.
Though the process must still be fine-tuned, the researchers have learned to control the size of the particles by varying the processing conditions. They are able to control the size such that only 4.5 percent of the nanocrystals produced are larger or smaller than the target size.
"In nanoparticle research, two things are important: size control and shape control," said Wang. "From a purity point of view, we have been able to obtain a high yield of nanocrystals whose shape was a real surprise."
Depending on conditions, the new nanocrystals can be as much as four times more catalytically active per unit area than existing commercial catalysts. But since the new structures tested are more than 20 times larger than existing platinum catalysts, they require more of the metal - and hence are less active per unit weight.
"We need to find a way to make these nanocrystals smaller while preserving the shape," Wang noted. "If we can reduce the size through better control of processing conditions, we will have a catalytic system that would allow production of hydrogen with greater efficiency."
Production of the new crystals begins with polycrystalline platinum spheres about 750 nanometers in diameter that are electrodeposited onto a substrate of amorphous - also known as "glassy" - carbon. Placed in an electrochemical cell with ascorbic acid and sulfuric acid, the spheres are then subjected to "square wave" potential that alternates between positive and negative potentials at a rate of 10 to 20 Hertz.
The electrochemical oxidation-reduction reaction converts the spheres to smaller nanocrystals over a period of time ranging from 10 to 60 minutes. The role of the carbon substrate isn't fully understood, but it somehow enhances the uniformity of the nanocrystals.
"The key to producing this shape is to tune the voltage and the time period under which it is applied," Sun noted. "By changing the experimental conditions, we can control the size with a high level of uniformity."
Scanning electron microscopy shows that the sizes average 81 nanometers in diameter, with the smallest just 20 nanometers. The microscopy also found that the structures were composed of single crystals with no dislocations.
"Not only do we have a beautiful shape - which was observed for the first time in this research - but we also have a very valuable catalyst," Sun added. "And because these nanocrystals are stable, the shape is preserved after the catalytic reaction, which will allow us to use the same nanocrystals over and over again."
In addition to Sun and Wang, the research team included Na Tian and Zhi-You Zhou from the College of Chemistry and Engineering at Xiamen University in China and Yong Ding from the School of Materials Science and Engineering at Georgia Tech in the United States.
Technical contacts: Zhong Lin Wang (404-894-8008); E-mail: ( ) or Shi-Gang Sun; E-mail: ( ).
####
About Georgia Institute of Technology Research News
The Georgia Institute of Technology is one of the nation's top research universities, distinguished by its commitment to improving the human condition through advanced science and technology.
Georgia Tech's campus occupies 400 acres in the heart of the city of Atlanta, where more than 16,000 undergraduate and graduate students receive a focused, technologically based education.
Accredited by the Southern Association of Colleges and Schools (SACS), the Institute offers many nationally recognized, top-ranked programs. Undergraduate and graduate degrees are offered in the Colleges of Architecture, Engineering, Sciences, Computing, Management, and the Ivan Allen College of Liberal Arts. Georgia Tech consistently ranks among U.S. News & World Report's top ten public universities in the United States. In a world that increasingly turns to technology for solutions, Georgia Tech is using innovative teaching and advanced research to define the technological university of the 21st century.
For more information, please click here
Contacts:
John Toon
404-894-6986
Copyright © Georgia Institute of Technology Research News
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








