Home > Press > Atomic force microscope reveals molecular ghosts: Mapping molecules with atomic precision expands toolbox for designing new catalytic reactions
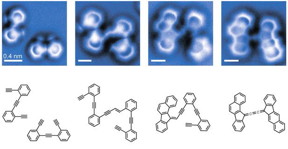 |
| An atomic force microscope was able to take a snapshot of the atoms before and after the reaction, but also found two supposedly short-lived intermediates (center) in this reaction of two enediyne molecules. CREDIT: UC Berkeley |
Abstract:
To the surprise of chemists, a new technique for taking snapshots of molecules with atomic precision is turning up chemicals they shouldn't be able to see.
Atomic force microscope reveals molecular ghosts: Mapping molecules with atomic precision expands toolbox for designing new catalytic reactions
Berkeley, CA | Posted on May 11th, 2016Chemical reactions take place so rapidly - often within picoseconds, or a trillionth of a second - that chemists expect intermediate steps in the reaction to be too brief to observe. Only lasers firing in femtosecond bursts - like a strobe flashing every thousandth of a picosecond - can capture the fleeting molecular structures that reacting chemicals form on their way to a final product.
Yet a team of chemists and physicists from the University of California, Berkeley, and Lawrence Berkeley National Laboratory has taken snapshots of two molecules reacting on the surface of a catalyst, and found intermediate structures lasting for the 20 minutes or so it takes to snap a photo.
"Intuitively, we did not expect to see these transient intermediates, because they are so short lived," said Felix Fischer, an assistant professor of chemistry at UC Berkeley. "Based on our traditional understanding, you would expect to see the starting materials and very shortly after, only the product. But we see these intermediates, so something else is going on."
The explanation for these ghostly molecules is now fleshing out details of catalytic reactions that chemists have only vaguely understood until now, and providing new rules for chemical reactions that chemists can exploit to make reactions go faster or more efficiently, or build molecules never before seen.
Fischer himself is just beginning to build a toolbox that will help design or improve catalytic reactions, which are the workhorse of the world's chemical industry, responsible for producing everything from fuel to the building blocks of plastics. These tools could also impact fields such as materials science, nanotechnology, biology and medicine.
"The way chemists think about heterogeneous catalysis appears to be an incomplete picture of what is actually happening on the surface," he said. "If we can understand how to take this tool box and use it in the design of new structures or the synthesis of new materials, that opens a whole new field of chemistry that so far has been dark to us, because we did not know how to actually visualize what is going on."
A paper describing their work appeared online this week in advance of publication in the journal Nature Chemistry.
Atomic force microscopy
Because chemical reactions occur so rapidly, chemists can only infer how chemicals change during the process, as bonds between atoms break and reform, branches rotate or join to form rings, and three-dimensional structures shift. Three years ago, Fischer and UC Berkeley's Michael Crommie, professor of physics, teamed up to apply the atom-scale precision of atomic force microscopy to take snapshots of molecules before and after a reaction, trying to confirm what chemists have always inferred.
Their non-contact atomic force microscope, or nc-AFM, hovers above a surface and detects individual atoms via a microscopic vibrating probe with a sensitive carbon monoxide molecule at its tip. Fischer, Crommie and their UC Berkeley colleagues place molecules on a gold or silver surface and heat them to make them react slowly, then use the nc-AFM to take snapshots over the course of the reaction.
During their first attempt to image a reaction between two molecules, they saw not only the starting chemicals and end product, but also two intermediate chemical structures that should not have been there. If you think of a reaction as a sequence of many intermediate chemical rearrangements, the easy structural changes should happen quickly while more complicated rearrangements would be slower, because there's a higher energy barrier to making those changes. But the intermediates he saw were ones that should have disappeared the fastest, based on current theories.
Organic chemists like Fischer tend to think of a chemical reaction as akin to falling downhill - once it starts, its own energy keeps it going until the final product appears. This concept didn't explain his results, however, so he borrowed an idea from chemical engineers who work with catalysts. To them, some intermediate states are bound more closely to the catalytic surface and lose energy to it, slowing the reaction. It's as if the reaction hit a rock on its downhill trajectory.
Fischer's colleague, Angel Rubio, director of the Max Planck Institute for the Structure and Dynamics of Matter in Hamburg and a professor at the University of the Basque Country in Spain, made extensive supercomputer calculations taking this surface binding into account, but still was not able to predict the intermediates actually observed.
Together they finally hit on the idea of taking into account the entropy changes at each step of the reaction, and matched observations exactly. Entropy - essentially the level of disorder or chaos in a system - hates to decrease, according to the third law of thermodynamics. So some transitions that seem energetically easy get stuck because they go from a flexible structure loosely bound to the catalyst - a high entropy situation - to a more rigid, tightly bound and lower-entropy situation.
"Taking entropy into account could help you understand the distribution of products you get from a heterogeneous catalysis reaction," he said. "It could help you predict which intermediates have a long lifetime on the surface, which ones could move around, adsorb or desorb from the surface, leading to a product distribution that might not be what you want. Then you could tune the reaction towards the product that you desire."
Fischer used his growing toolbox last year to make a molecule that was predicted more than half a century ago but unachievable using standard organic chemistry in solution. Instead, he built it on the surface of a catalyst from custom-made molecules that would normally not react in the right way, but which he guided to create an antiferromagnetic molecule called peripentacene.
"We used this toolbox of surface chemistry and the rules we have learned to make a molecule that no one had been able to make in 60 years," he said. "This is an example of why it is important to understand what is happening on these surfaces, and how you can use this understanding to access structures and reactivities that are not accessible with the standard tools we have right now."
###
Other co-authors of the Nature Chemistry paper are Alexander Riss, Sebastian Wickenburg, Hsin-Zon Tsai, Aaron Bradley, Miguel Ugeda, Han Sae Jung and Patrick Gorman of UC Berkeley, Alejandro Pérez Paz of the Universidad del País Vasco in Spain and Dimas G. De Oteyza of the Donostia International Physics Center in San Sebastián, Spain.
The work was funded by the Department of Energy, Office of Naval Research, European Research Council and Grupos Consolidados UPV/EHU del Gobierno Vasco.
####
For more information, please click here
Contacts:
Robert Sanders
510-643-6998
Copyright © University of California, Berkeley
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Physics
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Optically trapped quantum droplets of light can bind together to form macroscopic complexes March 8th, 2024
Optically trapped quantum droplets of light can bind together to form macroscopic complexes March 8th, 2024
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Military
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() New chip opens door to AI computing at light speed February 16th, 2024
New chip opens door to AI computing at light speed February 16th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








