Home > Press > Visualizing the Lithiation of a Nanosized Iron-Oxide Material in Real Time: Electron microscopy technique reveals the reaction pathways that emerge as lithium ions are added to magnetite nanoparticles
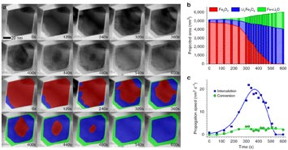 |
| (a) Bright-field scanning transmission electron microscope image series, which shows the three phases of lithiation over a 600-second period. Below the black-and-white images in the top two rows are falsely colored images that enhance the visualization of the different phases: pure magnetite (red), lithiated magnetite (blue), and metallic iron–lithium oxide composite (green). (b) The projected areas of the three phases in a single nanoparticle as a function of time. (c) The speeds of the intercalation and conversion reactions as functions of time. |
Abstract:
From cell phones to laptops and tablets, lithium-ion batteries power most of today's portable electronics. Understanding how these batteries store and release energy as they charge and discharge is critical to improving their performance and increasing their longevity, especially for high-power applications such as electric vehicles and smart power grids. Visualizing the atomic-scale reaction pathways involved in battery discharge, however, has been difficult because of the high sensitivity required to detect the corresponding local structural changes in battery materials just billionths of a meter in size.
Visualizing the Lithiation of a Nanosized Iron-Oxide Material in Real Time: Electron microscopy technique reveals the reaction pathways that emerge as lithium ions are added to magnetite nanoparticles
Upton, NY | Posted on May 9th, 2016Now, a team of scientists from the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, the University of Pennsylvania, and the University of Maryland, College Park, has developed an electron microscopy technique to visualize-in real time and at high resolution-such pathways. The scientists used this advanced technique, described in a Nature Communications paper published on May 9, to observe the discharge of a lithium-ion battery cell containing nanoparticles of magnetite-an inexpensive, nontoxic, high-conducting, high-energy-storage material. These discharge mechanisms were then correlated with the battery's discharge rates. The team's findings about how lithium migrates at the nanoscale could help improve the electrochemical performance of comparable electrode materials in lithium-ion batteries.
"Understanding how lithium ions penetrate and move in magnetite nanoparticles may help us to rationally design new nanoelectrodes for high-performance lithium-ion batteries," said Dong Su, a scientist in Brookhaven Lab's Center for Functional Nanomaterials, a DOE Office of Science User Facility, who led this research.
Imaging the lithiation of magnetite nanoparticles
To visualize how the structure of magnetite evolves during the discharge, or lithiation, process, the scientists used strain-sensitive, bright-field scanning transmission electron microscopy. In this technique, a "bright field" detector at the bottom of the microscope collects electrons transmitted through a sample, producing a contrast image in which regions with no sample in the electron beam path appear bright while thicker regions of the sample appear dark. The contrast of this image is sensitive to the strain, or the microforces, that produce very small local structural changes in a sample. In this case, the scientists inserted lithium ions into individual magnetite nanoparticles, observing how each nanoparticle's structure evolves throughout the phases of lithiation.
While the lithiation of magnetite and other metal oxides with similar structure is known to occur as a sequential two-step reaction of intercalation (insertion of lithium ions into compound) and conversion (decomposition of compound), the intercalation reaction had been impossible to visualize.
"During intercalation, the volume of the magnetite nanoparticle lattice volume changes only by a few percent because the inserted lithium ions simply fill empty spaces within the lattice. By comparison, conversion is much easier to see-there are no empty spaces to accommodate the lithium, so the lattice has no choice but to expand, actually breaking the electrode material in some cases," explained Su. "Our team is the first to capture the phase changes that occur in the nanoparticles during the intercalation reaction."
Determining the reaction pathways of lithiation
By analyzing the resulting microscope images, the scientists discovered that intercalation initially follows a two-phase "insertion and expansion" reaction sequence. Lithium ions first diffuse into the surface of the nanoparticle and then proceed inward. Under certain current conditions, further lithiation leads to the conversion reaction and the coexistence of three distinct phases within a single magnetite nanoparticle: pure magnetite (Fe3O4), lithiated rocksalt (LixFe3O4), and a composite of metallic iron (Fe) and lithium oxide (Li2O).
The team used an ex situ high-resolution transmission electron microscopy to track these atomic structural changes and to confirm they were not limited to a single nanoparticle but were characteristic of the entire battery cell. Patterns produced by the diffraction of X-rays on nanoparticle samples, an experiment conducted at the National Synchrotron Light Source II, a DOE Office of Science User Facility at Brookhaven Lab, verified the pure magnetite and lithiated rocksalt phases that occur during intercalation.
"This reaction inhomogeneity within a single particle means that intercalation and conversion are happening simultaneously in the middle course of the lithiation process," said Kai He, first author of this paper and former CFN postdoctoral researcher (now a research faculty member at Northwestern University). "The large lithium concentration at the particle surface could be triggering conversion early on while intercalation has not yet completed."
Given the laws of thermodynamics, the two reactions should occur at different voltages because of differences in their natural chemistry. The observed overlap between the two reactions suggests that the kinetic effect, or how charge or discharge currents impact the amount of energy that can be stored within a battery, plays an important role in lithiation.
At high discharge rates, for example, the intercalation reaction happens much faster than the conversion reaction. However, conversion accommodates more lithium ions because of the attachment sites made possible by the displacement of iron ions. So both reactions are important when considering the total lithium insertion capacity of a battery and, hence, its overall energy storage rate.
"The kinetic effect impacts the battery's performance. It is generally accepted that slowly charging a battery at a lower current maximizes energy capacity. But to optimize performance for high-power applications, we need to understand how phase evolution behaves with faster charge and discharge and figure out how to maximize these rates without sacrificing energy density," explained Christopher Murray, the Richard Perry University Professor of Chemistry and Materials Science and Engineering at the University of Pennsylvania, who is the co-corresponding author of the paper.
The team used computational modeling to describe the two-step reaction, calculating the discharge voltage at different lithium concentrations and simulating the lithiation process in magnetite nanoparticles. The simulation agreed with the real-time microscopy observation of mixed lithiation phases, with the voltage decreasing as conversion initiates.
In the future, the team hopes to develop a new method for simultaneously visualizing the phase evolution and measuring the corresponding electrochemical performance of electrode materials in real time.
"Our final goal is to find new electrode materials for lithium-ion batteries that can store higher amounts of charge and release energy more quickly than currently existing materials like graphite," said Sen Zhang, a NatureNet postdoctoral fellow on Murray's team. "By enabling us to understand the kinetic behavior of electrode materials at the nanoscale, our technique will help us reach this goal."
####
About Brookhaven National Laboratory
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Brookhaven National Laboratory is a multipurpose research institution funded by the U.S. Department of Energy. Located on Long Island, NY, Brookhaven operates large-scale facilities for studies in physics, chemistry, biology, medicine, applied science, and advanced technology. The Laboratory's almost 3,000 scientists, engineers, and support staff are joined each year by more than 5,000 visiting researchers from around the world.
For more information, please click here
Contacts:
Ariana Tantillo
(631) 344-2347
or
Peter Genzer
(631) 344-3174
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Magnetism/Magnons
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Videos/Movies
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
![]() Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Industrial
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
![]() Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








