Home > Press > Nanostructured metal-oxide catalyst efficiently converts CO2 to methanol: Highly reactive sites at interface of 2 nanoscale components could help overcome hurdle of using CO2 as a starting point in producing useful products
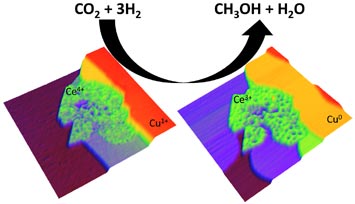 |
| Scanning tunneling microscope image of a cerium-oxide and copper catalyst (CeOx-Cu) used in the transformation of carbon dioxide (CO2) and hydrogen (H2) gases to methanol (CH3OH) and water (H2O). In the presence of hydrogen, the Ce4+ and Cu+1 are reduced to Ce3+ and Cu0 with a change in the structure of the catalyst surface. |
Abstract:
Scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory have discovered a new catalytic system for converting carbon dioxide (CO2) to methanol-a key commodity used to create a wide range of industrial chemicals and fuels. With significantly higher activity than other catalysts now in use, the new system could make it easier to get normally unreactive CO2 to participate in these reactions.
Nanostructured metal-oxide catalyst efficiently converts CO2 to methanol: Highly reactive sites at interface of 2 nanoscale components could help overcome hurdle of using CO2 as a starting point in producing useful products
Upton, NY | Posted on July 31st, 2014"Developing an effective catalyst for synthesizing methanol from CO2 could greatly expand the use of this abundant gas as an economical feedstock," said Brookhaven chemist Jose Rodriguez, who led the research. It's even possible to imagine a future in which such catalysts help mitigate the accumulation of this greenhouse gas, by capturing CO2 emitted from methanol-powered combustion engines and fuel cells, and recycling it to synthesize new fuel.
That future, of course, will be determined by a variety of factors, including economics. "Our basic research studies are focused on the science-the discovery of how such catalysts work, and the use of this knowledge to improve their activity and selectivity," Rodriguez emphasized.
The research team, which included scientists from Brookhaven, the University of Seville in Spain, and Central University of Venezuela, describes their results in the August 1, 2014, issue of the journal Science.
New tools for discovery
Because CO2 is normally such a reluctant participant in chemical reactions, interacting weakly with most catalysts, it's also rather difficult to study. These studies required the use of newly developed in-situ (or on-site, meaning under reaction conditions) imaging and chemical "fingerprinting" techniques. These techniques allowed the scientists to peer into the dynamic evolution of a variety of catalysts as they operated in real time. The scientists also used computational modeling at the University of Seville and the Barcelona Supercomputing Center to provide a molecular description of the methanol synthesis mechanism.
The team was particularly interested in exploring a catalyst composed of copper and ceria (cerium-oxide) nanoparticles, sometimes also mixed with titania. The scientists' previous studies with such metal-oxide nanoparticle catalysts have demonstrated their exceptional reactivity in a variety of reactions. In those studies, the interfaces of the two types of nanoparticles turned out to be critical to the reactivity of the catalysts, with highly reactive sites forming at regions where the two phases meet.
To explore the reactivity of such dual particle catalytic systems in converting CO2 to methanol, the scientists used spectroscopic techniques to investigate the interaction of CO2 with plain copper, plain cerium-oxide, and cerium-oxide/copper surfaces at a range of reaction temperatures and pressures. Chemical fingerprinting was combined with computational modeling to reveal the most probable progression of intermediates as the reaction from CO2 to methanol proceeded.
These studies revealed that the metal component of the catalysts alone could not carry out all the chemical steps necessary for the production of methanol. The most effective binding and activation of CO2 occurred at the interfaces between metal and oxide nanoparticles in the cerium-oxide/copper catalytic system.
"The key active sites for the chemical transformations involved atoms from the metal [copper] and oxide [ceria or ceria/titania] phases," said Jesus Graciani, a chemist from the University of Seville and first author on the paper. The resulting catalyst converts CO2 to methanol more than a thousand times faster than plain copper particles, and almost 90 times faster than a common copper/zinc-oxide catalyst currently in industrial use.
This study illustrates the substantial benefits that can be obtained by properly tuning the properties of a metal-oxide interface in catalysts for methanol synthesis.
"It is a very interesting step, and appears to create a new strategy for the design of highly active catalysts for the synthesis of alcohols and related molecules," said Brookhaven Lab Chemistry Department Chair Alex Harris.
###
The work at Brookhaven Lab was supported by the DOE Office of Science. The studies performed at the University of Seville were funded by the Ministerio de Economía y Competitividad of Spain and the European Regional Development Fund. The Instituto de Tecnologia Venezolana para el Petroleo supported part of the work carried out at the Central University of Venezuela.
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
####
About DOE/Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE's Office of Science by Brookhaven Science Associates, a limited-liability company founded by the Research Foundation for the State University of New York on behalf of Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit applied science and technology organization.
Visit Brookhaven Lab's electronic newsroom for links, news archives, graphics, and more at www.bnl.gov/newsroom, follow Brookhaven Lab on Twitter, twitter.com/BrookhavenLab, or find us on Facebook, www.facebook.com/BrookhavenLab/.
For more information, please click here
Contacts:
Karen McNulty Walsh
631-344-8350
Copyright © DOE/Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Thin films
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
![]() New study introduces the best graphite films: The work by Distinguished Professor Feng Ding at UNIST has been published in the October 2022 issue of Nature Nanotechnology November 4th, 2022
New study introduces the best graphite films: The work by Distinguished Professor Feng Ding at UNIST has been published in the October 2022 issue of Nature Nanotechnology November 4th, 2022
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Videos/Movies
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Industrial
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
![]() Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








