Home > Press > Up in Flames: Evidence Confirms Combustion Theory: Berkeley Lab and University of Hawaii research outlines the story of soot, with implications for cleaner-burning fuels
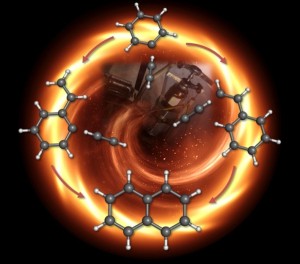 |
| Graphical representation of the chemistry in the early stages of soot formation. The mechanism to the right was demonstrated by experiment, while the one on the left was not. Credit: Dorian Parker, University of Hawaii |
Abstract:
Researchers at the Department of Energy's Lawrence Berkeley National Lab (Berkeley Lab) and the University of Hawaii have uncovered the first step in the process that transforms gas-phase molecules into solid particles like soot and other carbon-based compounds.
Up in Flames: Evidence Confirms Combustion Theory: Berkeley Lab and University of Hawaii research outlines the story of soot, with implications for cleaner-burning fuels
Berkeley, CA | Posted on July 1st, 2014The finding could help combustion chemists make more-efficient, less-polluting fuels and help materials scientists fine-tune their carbon nanotubes and graphene sheets for faster, smaller electronics. In addition, the results could have implications for the burgeoning field of astrochemistry, potentially establishing the chemical process for how gaseous outflows from stars turn into carbon-based matter in space.
"When you burn a flame, you start with a gas-phase reactant and then analyze the products, which include soot," says Musahid Ahmed, scientist in the Chemical Sciences Division at Berkeley Lab. "But there is no direct evidence for the chemical bonds that break and form in the process." For more than 30 years, scientists have developed computational models of combustion to explain how gas molecules form soot, but now Ahmed and his colleagues have data to confirm one long-standing theory in particular. "Our paper presents the first direct observation of this process," he says.
While the research is relevant to a number of disciplines—combustion science, materials science, and astrochemistry—it's combustion science that could see the most direct impact the soonest, says Ahmed. Specifically, the fundamental chemistry discovery could be used to find or design fuels that burn cleaner and don't produce as much soot.
Think about your car engine. If the combustion process were perfect, only carbon dioxide and water would come out of the tailpipe. Instead, we see fumes and particulates like soot, a visible macromolecule made up of sheets of carbon.
Theoretically, there are hundreds of different ways molecules can combine to create these dirty emissions. But there has been one popular class of mechanisms that outlines possible early steps for bond making and bond breaking during combustion. Called hydrogen abstraction-acetylene addition, or HACA, it was developed by Michael Frenklach professor of mechanical engineering at the University of California Berkeley in 1991.
One version of HACA works like this: during the high-temperature, high-pressure environment of combustion, a simple ring of six carbon and six hydrogen atoms, called benzene, would lose one of its hydrogen atoms, allowing another two-carbon molecule called acetylene, to attach to the ring, giving it a kind of tail. Then the acetylene tail would lose one of its hydrogen atoms so another acetylene could link up in, doubling the carbon atoms in the tail to four.
Next, the tail would curl around and attach to the original ring, creating a double-ring structure called naphthalene. Link by link, ring by ring, these molecules would continue to grow in an unwieldy, crumpled way until they became the macromolecules that we recognize as soot.
To test the first step of the theoretical HACA mechanism, Ahmed and collaborators from the University of Hawaii used a beamline at the Advanced Light Source (ALS) at Berkeley Lab specifically outfitted to study chemical dynamics. The ALS, a DOE Office of Science user facility, produces numerous photons over a wide range of energies, allowing researchers to probe a variety of molecules produced in this chemical reaction with specialized mass spectrometry analysis.
Unique to this experimental setup, Ahmed's team used a so-called hot nozzle, which recreates combustion environment in terms of pressure and temperature. The group started with a gaseous mix of nitrosobenzene (a benzene ring with a molecule of nitrogen and oxygen attached) and acetylene, and pumped it through a heated tube at a pressure of about 300 torr and a temperature of about 750 degrees Celsius. The molecules that came out the other end were immediately skimmed into a mass spectrometer that made use of the synchrotron light for analysis.
The researchers found two molecules predominantly emerged from the process. The more abundant kind was the carbon ring with a short acetylene tail on it, called phenylacetylene. But they also saw evidence for the double ring, naphthalene. These results, says Ahmed, effectively rule out one HACA mechanism—that a carbon ring would gain two separate tails and those tails would bond to form the double ring—and confirm the most popular HACA mechanism where a long tail curls around to form naphthalene.
Ahmed's local team included Tyler Troy, postdoctoral fellow at Berkeley Lab, and this work was performed with long-term collaborator Ralf Kaiser, professor of physical chemistry at the University of Hawaii at Manoa, and Dorian Parker, postdoctoral fellow also at Hawaii. The research was published June 20 online in the journal Angewandte Chemie.
"Having established the route to naphthalene, the simplest polycyclic aromatic hydrocarbon, the next step will be to unravel the pathways to more complex systems," says Kaiser.
Further experiments will investigate these follow-up mechanisms. It's a tricky feat, explains Ahmed, because the molecular possibilities quickly multiply. The researchers will add infrared spectroscopy to their analysis in order to catch the variety of molecules that form during these next phases of combustion.
This research was funded by the DOE Office of Science.
####
About DOE/Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
The DOE Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
For more information, please click here
Contacts:
Kate Greene
510-486-4404
Copyright © DOE/Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() The chemical dynamics beamline can be viewed on the web at:
The chemical dynamics beamline can be viewed on the web at:
![]() Snapshots of Ahmed’s science are available at:
Snapshots of Ahmed’s science are available at:
![]() Prof. Kaiser’s homepage is here:
Prof. Kaiser’s homepage is here:
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Graphene/ Graphite
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Aerospace/Space
![]() Under pressure - space exploration in our time: Advancing space exploration through diverse collaborations and ethical policies February 16th, 2024
Under pressure - space exploration in our time: Advancing space exploration through diverse collaborations and ethical policies February 16th, 2024
![]() Bridging light and electrons January 12th, 2024
Bridging light and electrons January 12th, 2024
![]() Manufacturing advances bring material back in vogue January 20th, 2023
Manufacturing advances bring material back in vogue January 20th, 2023
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








