Home > Press > Batteries as they are meant to be seen: In the search for long-lasting, inexpensive rechargeable batteries, researchers develop more realistic methods to study the materials in action
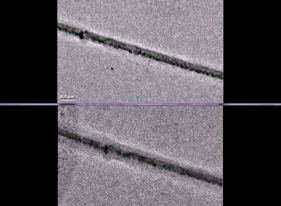 |
| Liquid battery electrolytes makes this view of an uncharged electrode (top) and a charged electrode (bottom) a bit fuzzy. Image courtesy of Gu et al, Nano Letters 2013 |
Abstract:
Researchers have developed a way to microscopically view battery electrodes while they are bathed in wet electrolytes, mimicking realistic conditions inside actual batteries. While life sciences researchers regularly use transmission electron microscopy to study wet environments, this time scientists have applied it successfully to rechargeable battery research.
Batteries as they are meant to be seen: In the search for long-lasting, inexpensive rechargeable batteries, researchers develop more realistic methods to study the materials in action
Richland, WA | Posted on December 27th, 2013The results, reported in December 11's issue of Nano Letters, are good news for scientists studying battery materials under dry conditions. The work showed that many aspects can be studied under dry conditions, which are much easier to use. However, wet conditions are needed to study the hard-to-find solid electrolyte interphase layer, a coating that accumulates on the electrode's surface and dramatically influences battery performance.
"The liquid cell gave us global information about how the electrodes behave in a battery environment," said materials scientist Chongmin Wang of the Department of Energy's Pacific Northwest National Laboratory. "And it will help us find the solid electrolyte layer. It has been hard to directly visualize in sufficient detail."
Ebb, Flow, Swell
Even though electricity seems invisible, storing and using it in batteries has some very physical effects. Charging a battery jams electrons into the negative electrode, where positively charged lithium ions (or another metal ion such as sodium) rush in to meet and hold onto the electrons. Those ions have to fit within pores within the electrode.
Powering a device with a battery causes the electrons to stream out of the electrode. The positive ions, left behind, surge through the body of the battery and return to the positive electrode, where they await another charging.
Wang and colleagues have used high-powered microscopes to watch how the ebbing and flowing of positively charged ions deform electrodes. Squeezing into the electrode's pores makes the electrodes swell, and repeated use can wear them down. For example, recent work funded through the Joint Center for Energy Storage Research — a DOE Energy Innovation Hub established to speed battery development — showed that sodium ions leave bubbles behind, potentially interfering with battery function.
But up to this point, the transmission electron microscopes have only been able to accommodate dry battery cells, which researchers refer to as open cells. In a real battery, electrodes are bathed in liquid electrolytes that provide an environment ions can easily move through.
So, working with JCESR colleagues, Wang led development of a wet battery cell in a transmission electron microscope at EMSL, the DOE's Environmental Molecular Sciences Laboratory on the PNNL campus. The team built a battery so small that several could fit on a dime. The battery had one silicon electrode and one lithium metal electrode, both contained in a bath of electrolyte.
Mystery Layer
When the team charged the battery, they saw the silicon electrode swell, as expected. However, under dry conditions, the electrode is attached at one end to the lithium source — and swelling starts at just one end as the ions push their way in, creating a leading edge. In this study's liquid cell, lithium could enter the silicon anywhere along the electrode's length. The team watched as the electrode swelled all along its length at the same time.
"The electrode got fatter and fatter uniformly. This is how it would happen inside a battery," said Wang.
The total amount the electrode swelled was about the same, though, whether the researchers set up a dry or wet battery cell. That suggests researchers can use either condition to study certain aspects of battery materials.
"We have been studying battery materials with the dry, open cell for the last five years," said Wang. "We are glad to discover that the open cell provides accurate information with respect to how electrodes behave chemically. It is much easier to do, so we will continue to use them."
As far as the elusive solid electrolyte interphase layer goes, Wang said they couldn't see it in this initial experiment. In future experiments, they will try to reduce the thickness of the wet layer by at least half to increase the resolution, which might provide enough detail to observe the solid electrolyte interphase layer.
"The layer is perceived to have peculiar properties and to influence the charging and discharging performance of the battery," said Wang. "However, researchers don't have a concise understanding or knowledge of how it forms, its structure, or its chemistry. Also, how it changes with repeated charging and discharging remains unclear. It's very mysterious stuff. We expect the liquid cell will help us to uncover this mystery layer."
This work was supported by the Department of Energy's Offices of Science and of Energy Efficiency and Renewable Energy.
####
About DOE/Pacific Northwest National Laboratory
The Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.
The Joint Center for Energy Storage Research (JCESR) is a major partnership that integrates researchers from many disciplines to overcome critical scientific and technical barriers and create new breakthrough energy storage technology. Led by the U.S. Department of Energy's Argonne National Laboratory, partners include national leaders in science and engineering from academia, the private sector, and national laboratories. Their combined expertise spans the full range of the technology-development pipeline from basic research to prototype development to product engineering to market delivery. Funding for JCESR is provided by the U.S. Department of Energy Office of Science.
EMSL, the Environmental Molecular Sciences Laboratory, is a national scientific user facility sponsored by the Department of Energy's Office of Science. Located at Pacific Northwest National Laboratory in Richland, Wash., EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. Its integrated computational and experimental resources enable researchers to realize important scientific insights and create new technologies. Follow EMSL on Facebook, LinkedIn and Twitter.
Contacts:
Mary Beckman
509-375-3688
Copyright © DOE/Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Imaging
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Tools
![]() Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response November 3rd, 2023
![]() The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
The USTC realizes In situ electron paramagnetic resonance spectroscopy using single nanodiamond sensors November 3rd, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








