Home > Press > Study shows how water dissolves stone, molecule by molecule: International team uses computers, experiments to better predict chemical dissolution
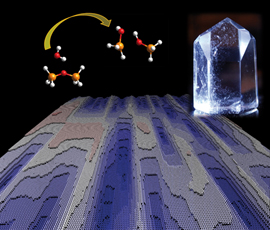 |
| The dissolution process of a crystalline structure in water is shown: two bonded SiO4 -- molecules dissolve (top left), a quartz crystal (top right) and the computer-simulated surface of a dissolving crystalline structure (below). CREDIT: MARUM & Rice University |
Abstract:
Scientists from Rice University and the University of Bremen's Center for Marine Environmental Sciences (MARUM) in Germany have combined cutting-edge experimental techniques and computer simulations to find a new way of predicting how water dissolves crystalline structures like those found in natural stone and cement.
Study shows how water dissolves stone, molecule by molecule: International team uses computers, experiments to better predict chemical dissolution
Houston, TX | Posted on December 5th, 2013In a new study featured on the cover of the Nov. 28 issue of the Journal of Physical Chemistry C, the team found their method was more efficient at predicting the dissolution rates of crystalline structures in water than previous methods. The research could have wide-ranging impacts in diverse areas, including water quality and planning, environmental sustainability, corrosion resistance and cement construction.
"We need to gain a better understanding of dissolution mechanisms to better predict the fate of certain materials, both in nature and in man-made systems," said lead investigator Andreas Lüttge, a professor of mineralogy at MARUM and professor emeritus and research professor in Earth science at Rice. His team specializes in studying the thin boundary layer that forms between minerals and fluids.
Boundary layers are ubiquitous in nature; they occur when raindrops fall on stone, water seeps through soil and the ocean meets the sea floor. Scientists and engineers have long been interested in accurately explaining how crystalline materials, including many minerals and stones, interact with and are dissolved by water. Calculations about the rate of these dissolution processes are critical in many fields of science and engineering.
In the new study, Lüttge and lead author Inna Kurganskaya, a research associate in Earth science at Rice, studied dissolution processes using quartz, one of the most common minerals found in nature. Quartz, or silicon dioxide, is a type of silicate, the most abundant group of minerals in Earth's crust.
At the boundary layer where quartz and water meet, multiple chemical reactions occur. Some of these happen simultaneously and others take place in succession. In the new study, the researchers sought to create a computerized model that could accurately simulate the complex chemistry at the boundary layer.
"The new model simulates the dissolution kinetics at the boundary layer with greater precision than earlier stochastic models operating at the same scale," Kurganskaya said. "Existing simulations rely on rate constants assigned to a wide range of possible reactions, and as a result, the total material flux from the surface have an inherent variance range -- a plus or minus factor that is always there."
One reason the team's simulations more accurately represent real processes is that its models incorporate actual measurements from cutting-edge instruments and from high-tech materials, including glass ceramics and nanomaterials. With a special imaging technique called "vertical scanning interferometry," which the group at MARUM and Rice helped to develop, the team scanned the crystal surfaces of both minerals and manufactured materials to generate topographic maps with a resolution of a just a few nanometers, or billionths of a meter.
"We found that dissolution rates that were predicted using rate constants were sometimes off by as much as two orders of magnitude," Lüttge said.
The new method for more precisely predicting dissolution processes could revolutionize the way engineers and scientists make many calculations related to a myriad of things, including the stability of building materials, the longevity of materials used for radioactive waste storage and more, he said.
"Further work is needed to prove the broad utility of the method," he said. "In the next phase of research, we plan to test our simulations on larger systems and over longer periods."
The research was supported by the Global Climate and Energy Project at Stanford University.
####
About Rice University
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,708 undergraduates and 2,374 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 2 for "best value" among private universities by Kiplinger's Personal Finance. To read "What they're saying about Rice," go to tinyurl.com/AboutRiceU.
Follow Rice News and Media Relations via Twitter @RiceUNews.
For more information, please click here
Contacts:
David Ruth
713-348-6327
Jade Boyd
713-348-6778
Copyright © Rice University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() A copy of the Journal of Physical Chemistry C paper is available at:
A copy of the Journal of Physical Chemistry C paper is available at:
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Videos/Movies
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
![]() Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Materials/Metamaterials/Magnetoresistance
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








