Home > Press > A cellular housekeeper, and potential target of obesity drugs, caught in action
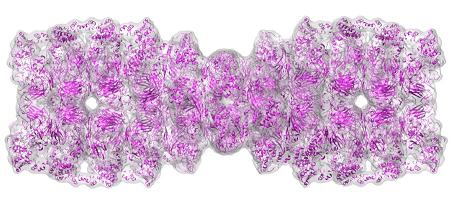 |
| New clues emerge about how a molecular machine breaks down unwanted proteins in cells thanks to work conducted at Berkeley Lab's Advanced Light Source. In this atomic-scale model of the molecular machine, tripeptidyl peptidase II, the cyan ribbon depicts the skeleton of the giant molecule. The grey enclosure represents the lower resolution surface and is included to aid visualization. |
Abstract:
Scientists from the U.S. Department of Energy's Lawrence Berkeley National Laboratory have obtained the closest look yet of how a gargantuan molecular machine breaks down unwanted proteins in cells, a critical housekeeping chore that helps prevent diseases such as cancer.
A cellular housekeeper, and potential target of obesity drugs, caught in action
Berkeley, CA | Posted on August 2nd, 2010They pieced together the molecular-scale changes the machine undergoes as it springs into action, ready to snip apart a protein.
Their work provides valuable clues as to how the molecular machine, a giant enzyme called tripeptidyl peptidase II, keeps cells tidy and disease free. It could also inform the development of obesity-fighting drugs. A closely related enzyme in the brain can cause people to feel hungry even after they eat a hearty meal.
"We can now better understand how this very important enzyme carries out its work, which has not been described at a molecular scale until now," says Bing Jap, a biophysicist in Berkeley Lab's Life Sciences Division. He led the research with scientists from the University of California at Berkeley and Germany's Max Planck Institute of Biochemistry.
The scientists report their research August 1 in an advance online publication of the journal Nature Structural & Molecular Biology.
Tripeptidyl peptidase II is found in all eukaryotic cells, which are cells that a have membrane-bound nucleus. Eukaryotic cells make up plants and animals. The enzyme's chief duty is to support the pathway that ensures that cells remain healthy and clutter free by breaking down proteins that are misfolded or have outlived their usefulness.
It's not always so helpful, however. A variation of the enzyme in the brain degrades a hormone that makes people feel satiated after a meal. When this hormone becomes unavailable, a person can eat and eat without feeling full, which can lead to obesity.
Tripeptidyl peptidase II is also the largest protein-degrading enzyme, or protease, in eukaryotic cells. It's more than 100 times larger than most other proteases.
Scientists don't know how this behemoth of an enzyme targets and degrades specific proteins — but it's good that the enzyme is so selective. If it degraded every protein it comes across, the cell would quickly die.
"We want to know how it's regulated, how it selects proteins to degrade, and how it cuts them apart," says Jap.
To help answer these questions, his team determined the changes the molecular machine undergoes as it readies itself for action. Using x-ray crystallography, they obtained an atomic-scale resolution structure of the molecular machine in its inactive state. This work was conducted at Berkeley Lab's Advanced Light Source, a national user facility that generates intense x-rays to probe the fundamental properties of substances.
They also developed a lower-resolution, three-dimensional map of the molecular machine in its activated state, meaning it's poised to snip apart a protein. This structure was determined using cryo-electron microscopy.
They then merged these two structures together, one dormant and the other ready to pounce on a protein.
"When we dock these structures, we can begin to ascertain the changes the enzyme undergoes as it transitions from an inactive to an active state," says Peter Walian, a scientist in Berkeley Lab's Life Sciences Division who also contributed to the research.
This first molecular-scale vantage of the enzyme in action offers insights into how it works. For example, the scientists found that only very small proteins can fit in the chamber the enzyme uses to break down proteins.
"This sheds light on how the enzyme targets specific proteins," says Jap.
They also learned more about how the enzyme uses a molecular ruler to mince proteins into pieces that only span three residues.
"This work is yielding valuable clues as to how the giant enzyme carries out very fundamental biological processes, with more insights to come," says Jap. "The obesity-related hormone is one of many interesting targets of the protease. There are likely other proteins and peptides, yet to be discovered, that are processed by this protease."
The research was supported by the National Institute of General Medical Sciences of the National Institutes of Health. The Advanced Light Source is supported by the Department of Energy's Office of Science.
Additional information:
The paper describing this work, titled, "Hybrid Molecular Structure of the Giant Protease Tripeptidyl Peptidase II," appears August 1, 2010 in an advance online publication of the journal Nature Structural & Molecular Biology.
####
About Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory provides solutions to the world’s most urgent scientific challenges including clean energy, climate change, human health, and a better understanding of matter and force in the universe. It is a world leader in improving our lives and knowledge of the world around us through innovative science, advanced computing, and technology that makes a difference. This content is solely the responsibility of Lawrence Berkeley National Laboratory. Berkeley Lab is a U.S. Department of Energy (DOE) national laboratory managed by the University of California for the DOE Office of Science.
For more information, please click here
Contacts:
Dan Krotz
(510) 486-4019
Copyright © Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Molecular Machines
![]() First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
![]() Nanotech scientists create world's smallest origami bird March 17th, 2021
Nanotech scientists create world's smallest origami bird March 17th, 2021
![]() Giant nanomachine aids the immune system: Theoretical chemistry August 28th, 2020
Giant nanomachine aids the immune system: Theoretical chemistry August 28th, 2020
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Nanobiotechnology
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








