Home > Press > Enzyme Detectives Uncover New Reactions, Products: Implications for engineering biofuels and plant-derived replacements for petro-chemicals
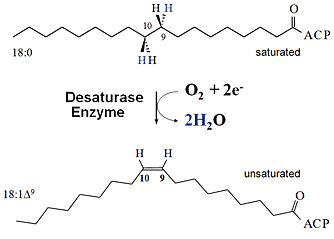 |
| Desaturation of Fatty Acids -- introducing a double bond turns bad fat into good fat. |
Abstract:
If your experiment doesn't go the way you expect, take a closer look — something even more interesting may have happened. That strategy has led scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory to discover a fundamental shift in an enzyme's function that could help expand the toolbox for engineering biofuels and other plant-based oil products. The results will be published online the week of September 8, 2008, in the Proceedings of the National Academy of Sciences.
Enzyme Detectives Uncover New Reactions, Products: Implications for engineering biofuels and plant-derived replacements for petro-chemicals
UPTON, NY | Posted on September 8th, 2008 The Brookhaven scientists were trying to understand the factors that affect where carbon-carbon double bonds are placed in fatty acids, the building blocks of oils and fats, when they are "desaturated" — that is, when a desaturase enzyme removes hydrogen from the carbon chain.
"Placing double bonds in different positions allows you to change the structure of the fatty acids to make products with different potential applications," explained Brookhaven biochemist John Shanklin, who led the research. The ultimate goal: engineering designer plant oils to be used as biofuels and/or raw materials to reduce the use of petroleum.
To try to change the position of a double bond, the Brookhaven team modified a desaturase enzyme, changing three of the 363 amino acids in its protein sequence. But when they tested the modified enzyme and looked for the expected product with its altered double-bond position, it wasn't there.
They could have moved on and made different amino acid changes to accomplish the initial goal. But Brookhaven research associate Edward Whittle was determined to figure out what was going on with the unusual result. "The substrate, or starting material, had been used up, so something was being produced — substrates can't just disappear," Whittle said. "If it wasn't the product we were looking for, what was it?"
Whittle's detective work uncovered a remarkable discovery. Instead of producing a shift in double-bond position, the enzyme modification had yielded three completely new products — two variations of a hydroxylated product called an allylic alcohol and a fatty acid containing two double bonds. "This was a profound shift in enzyme function," noted Shanklin, who has been working with modified enzymes for 15 years. "Usually you make changes very gradually, getting a few percent of a new product mixed with the original product. This was more like throwing a switch, making the change in function close to complete."
The discovery is also notable because the starting enzyme, like other soluble (membrane-independent) desaturases, can ordinarily perform only its one specified reaction — desaturation. This is unlike desaturase enzymes that reside within the cell membrane, which appear to be more versatile, performing a range of reactions. The soluble and membrane enzymes, however, do share one key feature: both perform reactions that require the production of a highly reactive form of oxygen.
"Since both classes of enzymes produce activated oxygen, in theory the soluble enzymes, like their membrane counterparts, should be able to perform a variety of reactions as well," Shanklin said. "Our work demonstrates that this is indeed the case. Making small changes to the enzyme's amino acid sequence has unlocked the soluble desaturase's potential to facilitate a wider range of chemistry than has been seen before," Shanklin said.
The challenge is to figure out how these structural changes to the enzyme lead to the observed changes in reaction chemistry. Computer-generated models combining the known structure of the starting enzyme in conjunction with its new substrates are helping the scientists understand how the enzyme works. The next step is to obtain real 3-D crystal structures of enzyme-substrate complexes, using the National Synchrotron Light Source at Brookhaven Lab, to see how they match up with the predictions.
Analyzing the structures of soluble enzymes is much simpler than obtaining structures for membrane enzymes. So, in effect, this work is a fast-track approach for correlating structure with function, which should help scientists gain general mechanistic insights relevant to both classes of enzymes. "Understanding how nature has figured out how to do this very difficult chemistry, and how to control that chemistry," Shanklin said, "would be extremely satisfying from a purely scientific perspective. But applying this knowledge could have benefits for us all."
"Right now, the materials we use — the plastics, foams, nylons — have been limited by the structures of petroleum-based chemical feedstocks. But if we understand how to engineer designer desaturase-like plant enzymes, we can tailor-make feedstocks with optimal properties, instead of relying on the properties of preexisting raw materials," said Shanklin. "We'd no longer have to say, ‘this is what we have so this is what we can make.' Instead, we could make the best feedstock for a particular application by designing the raw materials that will yield it."
This research was funded by the Office of Basic Energy Sciences within DOE's Office of Science, and by the Natural Sciences and Engineering Research Council of Canada. In addition to Shanklin and Whittle, the research team includes Amy Tremblay and Peter Buist of Carleton University in Ottawa, Canada.
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE’s Office of Science by Brookhaven Science Associates, a limited-liability company founded by Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization.
For more information, please click here
Contacts:
Karen McNulty Walsh
(631) 344-8350
or
Mona S. Rowe
(631) 344-5056
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Videos/Movies
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
![]() Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Visualizing the invisible: New fluorescent DNA label reveals nanoscopic cancer features March 4th, 2022
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








