Home > Press > Brown Chemist Finds Platinum Nanocube Enhances Fuel Cell Operation
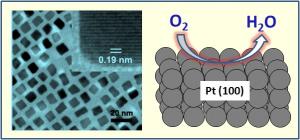 |
| The Making of a Platinum Nanocube On the left is a transmission electron microscopy image of 7 nanometer platinum nanocubes used for oxygen reduction reaction. In the upper right corner of this image is a high resolution picture of a single cube. On the right is an illustration demonstrating the oxygen reduction on a Pt(100) surface of a cube. Credit: Courtesy of Chao Wang/Brown University |
Abstract:
A team of chemists at Brown University for the first time has consistently created uniform platinum nanocubes, a breakthrough that could make hydrogen fuel cells more efficient and less costly.
Brown Chemist Finds Platinum Nanocube Enhances Fuel Cell Operation
Providence, RI | Posted on April 21st, 2008Two great obstacles to hydrogen-powered vehicles lie with fuel cells. Fuel cells, which like batteries produce electrical power through chemical reactions, have been plagued by their relatively low efficiency and high production costs. Scientists have tested a wide assortment of metals and materials to overcome the twin challenge.
Now a team led by Brown University chemistry Professor Shouheng Sun has mastered a Rubik's Cube-like dilemma for dealing with platinum, a precious metal coveted for its ability to boost a chemical reaction in fuel cells. They show that shaping platinum into a cube greatly enhances its efficiency in a phase of the fuel cell's operation known as oxygen reduction reaction. Sun's results have been published online in the journal Angewandte Chemie. The paper was selected as a Very Important Paper, reserved for less than five percent of manuscripts submitted to the peer-reviewed journal.
Platinum helps reduce the energy barrier - the amount of energy needed to start a reaction - in the oxidation phase of a fuel cell. It is also seen as beneficial on the other end of the fuel cell, known as the cathode. There, platinum has been shown to assist in oxygen reduction, a process in which electrons peeled from hydrogen atoms join with oxygen atoms to create electrical energy. The reaction also is important because it only produces water. This byproduct - rather than the global warming gas carbon dioxide - is a big reason why hydrogen fuel cells are a tantalizing area of research from carmakers in Detroit to policymakers in Washington.
But scientists have had trouble maximizing platinum's potential in the oxygen reduction reaction. The barriers chiefly revolve around shape and surface area - geometry and geography, so to speak. What Sun has learned is that molding platinum into a cube on the nanoscale enhances its catalysis - that is, it boosts the rate of a chemical reaction.
"For the first time, we can control the morphology of the particle to make it more like a cube," Sun said. "People have had very limited control over this process before. Now we have shown it can be done uniformly and consistently."
During his experiments, Sun, along with Brown graduate engineering student Chao Wang and engineers from the Japanese firm Hitachi Maxwell Ltd., created polyhedron and cube shapes of different sizes by adding platinum acetylacetonate (Pt(acac)2) and a trace amount of iron pentacarbonyl (Fe(CO)5) at specific temperature ranges. The team found that cubes were more efficient catalysts, owing largely to their surface structure and their resistance to being absorbed by the sulfate in the fuel cell solution.
"For this reaction, the shape is more important than the size," Sun said.
The next step, Sun added, is to build a polymer electrolyte membrane fuel cell and test the platinum nanocubes as catalysts in it. The team expects the experiments will yield fuel cells with a higher electrical output than previous versions.
"It's like science fiction, but we're a step closer now to the reality of developing a very efficient platinum catalyst for hydrogen cars that produce only water as exhaust," Sun said.
Hitachi Maxell chemical engineers Hideo Daimon, Taigo Ondera and Tetsunori Koda, a visiting engineer at Brown, contributed to the research.
The research was funded by the National Science Foundation and by the Office of the Vice President of Research at Brown University through its Research Seed Fund.
####
For more information, please click here
Contacts:
Richard Lewis
(401) 863-3766
Copyright © Brown University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








