Home > Press > Nanobiotix's promising data from Phase I/II head and neck cancer trial presented at ASCO
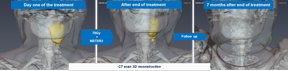 |
| Illustration : 3D reconstruction of CT scan (tumor in yellow) patient treated at a 15% dose level showing a Complete Response 7 months after the end of the treatment. |
Abstract:
· Very good safety profile with no AEs and SAEs in stage III/IV in frail patients older than 70 years old
· 7 out 9 patients had Complete Reponse at 10% dose level or more
· Follow up shows a potential impact on long term disease control
· Amendment filed for a dose expansion cohort of 44 additional patients
· Plan to open this study in the United States
Nanobiotix's promising data from Phase I/II head and neck cancer trial presented at ASCO
Paris, France and Cambridge, MA | Posted on June 5th, 2017 NANOBIOTIX (Euronext: NANO – ISIN: FR0011341205), a late clinical-stage nanomedicine company pioneering new approaches to the treatment of cancer, presented the results of the Phase I/II head and neck cancer trial with its lead product candidate, NBTXR3, at the American Society of Clinical Oncology (ASCO), Chicago.
Nanobiotix’s Chief Medical Officer Elsa Borghi said: “The very good level of tolerance seen so far, the absence of Adverse Events related to the product, and the rate of Complete Response in this population, indicates that NBTRX3 could play a key role in the treatment of head and neck cancer. It could potentially have a direct impact on the patients’ outcome via Loco-regional Control, Quality of Life, Safety and Overall Survival.”
Population treated
Head and neck cancers represent a group of aggressive cancers that appear in the mouth, nose, sinuses and at the top of the aerodigestive tract. This disease is a major public health concern in USA, Europe and Asia.
Nanobiotix’s Phase I/II head and neck trial targets frail and elderly patients (more than 70 years) who have advanced stage III/IV cancer with very limited therapeutic options. The only available treatment for these patients is radiotherapy, as their condition does not allow them to receive a combination of radiotherapy and chemotherapy, which offers a better survival outcome. These patients with radiotherapy treatment alone have a poorer outcome with lower Response Rate, and shorter Overall Survival. Uncontrolled tumor growth in such population will significantly decrease patients’ Quality of Life because basic functions such as swallowing, breathing, speaking and eating are impaired.
The use of Nanobiotix’s NBTXR3 in this population aims to provide better local and systemic desease control and prolongs survival with the improvement of Quality of Life.
Results presented at ASCO annual meeting (Abstract #6080)
A phase I trial of NBTXR3 nanoparticles activated by intensity-modulated radiation therapy (IMRT) in the treatment of advanced-stage head and neck squamous cell carcinoma (HNSCC). (Poster board #68, Authors: Christophe Le Tourneau, MD, PhD, Valentin Calugaru, Thomas Jouffroy, Jose Rodriguez, Caroline Hoffmann, Bernard Dodger, Victor Moreno, Emiliano Calvo; Institut Curie, Paris, France; START Madrid, FJD, Madrid, Spain; Centro Integral Oncológico Clara Campal, Madrid, Spain).
1. Primary endpoints: Safety and Feasibility
NBTXR3 has demonstrated an excellent safety profile, with no Adverse Events (serious or not) related to the product.The radiotherapy safety observed in the trial has been strictly the same to the IMRT well-known toxicity. This is an important finding, given the elderly and frail population treated in this trial.
Additionally, the injection was demonstrated to be feasible and appropriate as the product remained in the tumor from the first day until the last day of radiotherapy. Marginal passage in the blood circulation has been observed during injection time. No leakage in the surrounding tissues have been observed. The highest dose (22%) continues to be evaluated.
2. Exploratory endpoints: efficacy and patient outcome (follow up and duration of Response)
The Overall Response Rate (Partial Response plus Complete Response) was evaluated as per RECIST 1.1.
The first data showed promising signs of antitumor activity. The Overall Response Rate is 91% (10 out of 11 patients evaluable) and 7 out 9 patients (78%) had Complete Reponse at 10% dose level or more. In addition, the tumor response suggests a dose dependent effect (see figure below: waterfall plot).
So far, all of the patients treated at higher dose levels (15% and 22%) have shown a prolongated Response with no loco-regional or distant relapse, with a medium follow up of 12 months.
Additional findings
The trial also showed that most of the Complete Responses occurred between 3 and 10 months after the end of the treatment, during the follow-up period when patients were not receiving any oncology treatments. Interestingly late appearance of tumor Complete Response as well as an unusual case of Pseudo Disease Progression followed by tumor Complete Response have been observed in the study.
Given the existing pre-clinical data and recent clinical data in Soft Tissue Sarcoma patients (abstract ASCO 2017 number e14615 http://bit.ly/2rsNi2M) showing the abiltity of NBTXR3 to trigger a specific adaptative immune pattern, Nanobiotix will include an immulogical biomarkers analysis in this study.
Potential Value of NBTXR3 in this indication
In oncology, finding ways to impact overall survival rate and quality of life with good safety is the ultimate goal. The preliminary findings seem to show that NBTXR3 has the potential to do this.
The excellent safety profile demonstrated thus far in this elderly and frail population, indicates that NBTXR3 would represent a valuable option to preserve and improve Quality of Life compared to other treatments. This safety profile also opens up opportunities for combinations with different types of treatment.
These encouraging results point towards a positive improvement of loco-regional Control, impacting Overall Survival.
Next steps
Nanobiotix is filing a protocol amendment of this study to include 44 additional patients in an expansion to demonstrate the efficacy of NBTXR3. Nanobiotix is opening 12-15 additional sites in Europe to expand the development of this indication. The company plans to expand this study in the US.
***
About NBTXR3 phase I/II trial in Head & Neck cancer
A significant proportion of head and neck carcinomas in the western world are found in the oral cavity, and the oropharynx, the posterior continuation of the oral cavity that connects with the nasopharynx (above) and laryngopharynx (below).
These structures play a crucial role in swallowing, breathing and speaking. Locally advanced oropharyngeal cancers can obstruct the airflow or infiltrate muscles or nerves, significantly disrupting essential local functions. Response in H&N cancer patients is related to: Age, stage, size, comorbidity, localization of the tumor and infection by the human papilloma virus (presence versus absence of HPV).
Local control of the tumor, when possible, is critical to preserve organ function, quality of life and has a direct impact on the disease outcome including Progression – Free Survival and (PFS) Overall Survival (OS).
Design
The target population for the Phase I/II trial are patients with locally advanced squamous cell carcinoma of the oral cavity, tongue or oropharynx (Stage T3 and T4), who are also classified as frail and elderly. They have a poorer prognosis as compared to other Head and Neck cancer patients. In this population tumor response and local control are usually very low compare to patients eligible for combined treatment: radiotherapy plus cisplatin.
This study has targeted patients with bulky tumors, with significant invasion of local tissues. In order to ensure the optimal treatment for every patient, the design of the study has included two routes of injection of NBTXR3: intratumoral injection and super selective intra-arterial injection.
Arm 1: Intra Tumoral (IT) injection, Dose escalation (5%, 10%, 15%, 22% of the tumor volume). Number of patients could go up to 20 (3 to 6 patients per dose level could be treated; 3 if no safety issues).
Arm 2: Intra Arterial (IA) injection, Dose escalation (5%, 10%, 15%, 22% of the tumor volume). Number of patients could go up to 20 (3 to 6 patients per dose level could be treated; 3 if no safety issues). Arm 2 has not been explored as the IT injection in arm 1 has been shown to be feasible and successful.
Patients received 35 daily sessions (2GY per session) of radiotherapy starting one day after the injection of NBTXR3 with a total of 70Gy (standard of care).
At 50Gy (71% of the total dose) tumor volume is evaluated to assess the possibility of the patient to continue RTx (if tumor volume shrinkage is more than 50%) and avoid further unnecessary radiation toxicity and salvage surgery.
####
About Nanobiotix
Nanobiotix (Euronext: NANO / ISIN: FR0011341205) is a late clinical-stage nanomedicine company pioneering novel approaches for the treatment of cancer. The Company’s first-in-class, proprietary technology, NanoXray, enhances radiotherapy energy with a view to provide a new, more efficient treatment for cancer patients.
NanoXray products are compatible with current radiotherapy treatments and are meant to treat potentially a wide variety of solid tumors including soft tissue sarcoma, head and neck cancers, liver cancers, prostate cancer, breast cancer, glioblastoma, etc., via multiple routes of administration.
NBTXR3 is being evaluated in: soft tissue sarcoma (STS), head and neck cancers, prostate cancer, and liver cancers (primary and metastases). Additionally, head and neck cancer and rectal cancer trials led by Nanobiotix’s Taiwanese partner, PharmaEngine, are underway in the Asia Pacific region. The Company has filed in August 2016 for market approval (CE Marking) in Europe for its lead product NBTXR3.
The Company started in 2016 a new preclinical research program in Immuno-oncology with its lead product NBTXR3, which could have the potential to bring a new dimension to cancer immunotherapies.
Nanobiotix is listed on the regulated market of Euronext in Paris (ISIN: FR0011341205, Euronext ticker: NANO, Bloomberg: NANO: FP). The Company Headquarter is based in Paris, France. Affiliate in Cambridge, United States.
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the update of the reference document of Nanobiotix filed with the French Financial Markets Authority (Autorité des Marchés Financiers) under number D.16-0732-A01 on December 27, 2016 (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country. At the moment NBTXR3 does not bear a CE mark and is not permitted to be placed on the market or put into service until NBTXR3 has obtained a CE mark.
For more information, please click here
Contacts:
Nanobiotix
Sarah Gaubert
Director, Communications & Public Affairs
+33 (0)1 40 26 07 55
/
Noël Kurdi
Director, Investor Relations
+1 (646) 241-4400
/
Media relations
France - Springbok Consultants
Marina Rosoff
+33 (0)6 71 58 00 34
United States – RooneyPartners
Marion Janic
+1 (212) 223-4017
Copyright © Nanobiotix
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Cancer
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Super-efficient laser light-induced detection of cancer cell-derived nanoparticles: Skipping ultracentrifugation, detection time reduced from hours to minutes! October 6th, 2023
Super-efficient laser light-induced detection of cancer cell-derived nanoparticles: Skipping ultracentrifugation, detection time reduced from hours to minutes! October 6th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Events/Classes
![]() Researchers demonstrate co-propagation of quantum and classical signals: Study shows that quantum encryption can be implemented in existing fiber networks January 20th, 2023
Researchers demonstrate co-propagation of quantum and classical signals: Study shows that quantum encryption can be implemented in existing fiber networks January 20th, 2023
Nanobiotechnology
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








