Home > Press > In water as in love, likes can attract
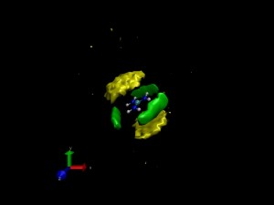 |
| This model of the guanidinium chloride salt (blue and silver) in solution shows carbon (yellow) and water (green) surrounding the cations and demonstrates cation-cation pairing. |
Abstract:
At some point in elementary school you were shown that opposite charges attract and like charges repel. This is a universal scientific truth - except when it isn't. A research team led by Berkeley Lab chemist Richard Saykally and theorist David Prendergast, working at the Advanced Light Source (ALS), has shown that, when hydrated in water, positively charged ions (cations) can actually pair up with one another.
In water as in love, likes can attract
Berkeley, CA | Posted on September 19th, 2013"Through a combination of X-ray spectroscopy, liquid microjets and first principles' theory, we've observed and characterized contact pairing between guanidinium cations in aqueous solution," Saykally says. "Theorists have predicted this cation-to-cation pairing but it has never been definitively observed before. If guanidinium cations can pair this way, then other similar cation systems probably can too."
Guanidinium is an ionic compound of hydrogen, nitrogen and carbon atoms whose salt - guanidinium chloride - is widely used by scientists to denature proteins for protein-folding studies. This practice dates back to the late 19th century when the Czech scientist Franz Hofmeister observed that cations such as guanidinium can pair with anions (negatively charged ions) in proteins to cause them to precipitate. The Hofmeister effect, which ranks ions on their ability to "salt-out" proteins, became a staple of protein research even though its mechanism has never been fully understood.
In 2006, Kim Collins of the University of Maryland proposed a "Law of Matching Water Affinities" to help explain "Hofmeister effects". Collins's proposal holds that the tendency of a cation and anion to form a contact pair is governed by how closely their hydration energies match, meaning how strongly the ions hold onto molecules of water. Saykally, who is a faculty scientist in Berkeley Lab's Chemical Sciences Division and a professor of chemistry at the University of California Berkeley, devised a means of studying both the Law of Matching Water Affinities and Hofmeister effects. In 2000, he and his group incorporated liquid microjet technology into the high-vacuum experimental environment of ALS beamlines and used the combination to perform the first X-ray absorption spectroscopy measurements on liquid samples. This technique has since become a widely used research practice.
"The XAS spectrum is generally sensitive to the changes in the local solvation environment around each atom, including potential effects of ion-pairing," Saykally says. "However, the chemical information that one can extract from such experimental data alone is limited, so we interpret our spectra with a combination of molecular dynamics simulations and a first principles theory method."
Development of this first principles theory method was led by Prendergast, a staff scientist in the Theory of Nanostructures Facility at Berkeley Lab's Molecular Foundry. Computational resources were provided by the National Energy Research Scientific Computing Center (NERSC). The Molecular Foundry and NERSC, as well as the ALS, are all U.S. Department of Energy national user facilities hosted at Berkeley Lab.
With the liquid microjet technology, a sample rapidly flows through a fused silica capillary shaped to a finely tipped nozzle with an opening only a few micrometers in diameter. The resulting liquid beam travels a few centimeters in a vacuum chamber and is intersected by an X-ray beam then collected and condensed out. In analyzing their current results, which were obtained at ALS Beamline 8.0.1, the Berkeley Lab researchers concluded that the counterintuitive cation-cation pairing observed is driven by water-binding energy, as predicted by theory.
Orion Shih, a recent graduate of Saykally's research group, is the lead author of a paper describing this study in the Journal of Chemical Physics. The paper is titled "Cation-cation contact pairing in water: Guanidinium." Saykally is the corresponding author. Other co-authors are Alice England, Gregory Dallinger, Jacob Smith, Kaitlin Duffey, Ronald Cohen and Prendergast.
"We found that the guanidinium ions form strong donor hydrogen bonds in the plane of the molecule, but weak acceptor hydrogen bonds with the pi electrons orthogonal to the plane," Shih says. "When fluctuations bring the solvated ions near each other, the van der Waals attraction between the pi electron clouds squeezes out the weakly held water molecules, which move into the bulk solution and form much stronger hydrogen bonds with other water molecules. This release of the weakly interacting water molecules results in contact pairing between the guanidinium cations. We believe our observations may set a general precedent in which like charges attract becomes a new paradigm for aqueous solutions."
####
For more information, please click here
Contacts:
Lynn Yarris
510-486-5375
Copyright © DOE/Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() For more about Richard Saykally and his research group go here:
For more about Richard Saykally and his research group go here:
![]() For more about Berkeley Lab’s Advanced Light Source go here:
For more about Berkeley Lab’s Advanced Light Source go here:
![]() For more about Berkeley Lab’s Molecular Foundry go here:
For more about Berkeley Lab’s Molecular Foundry go here:
| Related News Press |
Physics
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Focused ion beam technology: A single tool for a wide range of applications January 12th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








