Home > Press > A hot bath for gold nanoparticles
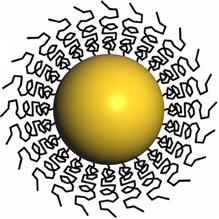 |
| A schematic diagram shows a gold nanoparticle stabilized with polyvinyl alcohol (PVA) ligands. |
Abstract:
Gold nanoparticles, says Chris Kiely, are fast becoming some of the most effective diplomats of the nanoworld.
They facilitate a wide range of chemical reactions between molecules that would not normally interact or would do so only at much higher temperatures.
And in most cases, they effect a single favorable outcome with few, if any, unwanted side reactions.
A hot bath for gold nanoparticles
Bethlehem, PA | Posted on August 2nd, 2011In short, says Kiely, a professor of materials science and engineering, the nanoparticles are extremely good catalysts.
Conventional methods of preparing gold nanoparticles, however, alter the morphology and catalytic activity of the particles.
Now, an international team of researchers has developed a procedure that enhances the surface exposure of gold nanoparticles and their catalytic activity over a range of reactions.
A new procedure improves on convention
The team reported its results in July in Nature Chemistry in an article titled "Facile removal of stabilizer-ligands from supported gold nanoparticles."
Its members include Kiely and Graham Hutchings, a chemist at Cardiff University in Wales in the U.K., who have studied nanogold together for more than a decade.
"In industry," says Kiely, "the most common way of preparing gold nanocatalysts is to first impregnate a nanocrystalline oxide support, such as titanium oxide (TiO2) with chloroauric acid. A reduction reaction then converts the acid into metal nanoparticles.
"Unfortunately, this leads to a variety of gold species being dispersed on the support, such as isolated gold atoms, mono- and bi-layer clusters, in addition to nanoparticles of various sizes."
An alternative technique that allows more precise control over particle size and structure, is to pre-form the gold nanoparticles in a colloidal solution before depositing them onto the support.
The disadvantage to this method is that during fabrication the nanoparticles are coated with organic molecules - ligands - that prevent them from clumping together. Once they are deposited onto a support, these ligands tend to impair the nanoparticle's catalytic performance by blocking the approach of molecules to active sites on the metal surface.
A milder form of ligand removal
Previous methods for stripping away these ligands have involved heat treatments of up to 400 degrees C.
"At these temperatures the morphology of the nanoparticles changes and they begin to coalesce," says Kiely. "There is also significant decrease in their catalytic activity."
The Kiely-Hutchings team developed a milder alternative for removing the ligands from polyvinyl alcohol-stabilized gold nanoparticles deposited on a titanium oxide support - a simple hot water wash.
Graduate student Ramchandra Tiruvalam used Lehigh's aberration-corrected JEOL 2200 FS transmission electron microscope to examine the catalysts before and after washing and to compare them with those that had undergone heat treatment to remove the ligands.
"Hot water washing had very little effect on particle size," says Kiely, who directs Lehigh's Nanocharacterization Laboratory, "and while the particles retain their cub-octahedral morphology, their surfaces appear to become more distinctly faceted. This is presumably due to some surface reconstruction occurring after losing a significant fraction of the protective PVA ligands."
"Heating the samples to 400 degrees C was also effective at removing the ligands but the average particle size increased from 3.7 to 10.4nm," says Kiely. "There was also tendency for the particles to restructure and develop flatter, more extended interfaces with the underlying TiO2 support."
For the oxidation of carbon monoxide to carbon dioxide, catalysts prepared by this colloidal/hot water wash displayed more than double the activity of conventional gold/TiO2 catalysts. This particular reaction is crucial for the removal of carbon monoxide from enclosed spaces such as submarines and space craft, prolonging the life of fuel cells, and extending the usable lifetime of a firefighter's mask.
This work was funded in part by the National Science Foundation. Tiruvalam is now a research scientist with Haldor Topsoe, a catalyst company in Copenhagen, Denmark.
####
About Lehigh University
Lehigh is a premier residential research university, ranked in the top tier of national research universities each year. We are a coeducational, nondenominational, private university that offers a distinct academic environment of undergraduate and graduate students from across the globe.
For more information, please click here
Contacts:
Carol Kiely
Copyright © Lehigh University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Military
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() New chip opens door to AI computing at light speed February 16th, 2024
New chip opens door to AI computing at light speed February 16th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Aerospace/Space
![]() Under pressure - space exploration in our time: Advancing space exploration through diverse collaborations and ethical policies February 16th, 2024
Under pressure - space exploration in our time: Advancing space exploration through diverse collaborations and ethical policies February 16th, 2024
![]() Bridging light and electrons January 12th, 2024
Bridging light and electrons January 12th, 2024
![]() Manufacturing advances bring material back in vogue January 20th, 2023
Manufacturing advances bring material back in vogue January 20th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








