Home > Press > Polymer membranes with molecular-sized channels assemble themselves
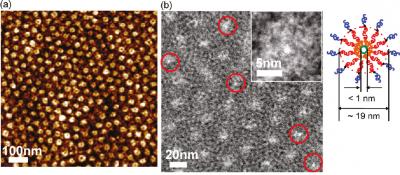 |
| Image (a) is an AFM image of a polymer membrane whose dark core corresponds to organic nanotubes. (b) is a TEM showing a sub-channeled membrane with the organic nanotubes circled in red. Inset shows zoomed-in image of a single nanotube. Credit: Image from Ting Xu |
Abstract:
Many futurists envision a world in which polymer membranes with molecular-sized channels are used to capture carbon, produce solar-based fuels, or desalinating sea water, among many other functions. This will require methods by which such membranes can be readily fabricated in bulk quantities. A technique representing a significant first step down that road has now been successfully demonstrated.
Polymer membranes with molecular-sized channels assemble themselves
Berkeley, CA | Posted on January 12th, 2011Researchers with the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley have developed a solution-based method for inducing the self-assembly of flexible polymer membranes with highly aligned subnanometer channels. Fully compatible with commercial membrane-fabrication processes, this new technique is believed to be the first example of organic nanotubes fabricated into a functional membrane over macroscopic distances.
"We've used nanotube-forming cyclic peptides and block co-polymers to demonstrate a directed co-assembly technique for fabricating subnanometer porous membranes over macroscopic distances," says Ting Xu, a polymer scientist who led this project. "This technique should enable us to generate porous thin films in the future where the size and shape of the channels can be tailored by the molecular structure of the organic nanotubes."
Xu, who holds joint appointments with Berkeley Lab's Materials Sciences Division and the University of California Berkeley's Departments of Materials Sciences and Engineering, and Chemistry, is the lead author of a paper describing this work, which has been published in the journal ACS Nano. The paper is titled "Subnanometer Porous Thin Films by the Co-assembly of Nanotube Subunits and Block Copolymers."
Co-authoring the paper with Xu were Nana Zhao, Feng Ren, Rami Hourani, Ming Tsang Lee, Jessica Shu, Samuel Mao, and Brett Helms, who is with the Molecular Foundry, a DOE nanoscience center hosted at Berkeley Lab.
Channeled membranes are one of nature's most clever and important inventions. Membranes perforated with subnanometer channels line the exterior and interior of a biological cell, controlling - by virtue of size - the transport of essential molecules and ions into, through, and out of the cell. This same approach holds enormous potential for a wide range of human technologies, but the challenge has been finding a cost-effective means of orienting vertically-aligned subnanometer channels over macroscopic distances on flexible substrates.
"Obtaining molecular level control over the pore size, shape, and surface chemistry of channels in polymer membranes has been investigated across many disciplines but has remained a critical bottleneck," Xu says. "Composite films have been fabricated using pre-formed carbon nanotubes and the field is making rapid progess, however, it still presents a challenge to orient pre-formed nanotubes normal to the film surface over macroscopic distances."
For their subnanometer channels, Xu and her research group used the organic nanotubes naturally formed by cyclic peptides - polypeptide protein chains that connect at either end to make a circle. Unlike pre-formed carbon nanotubes, these organic nanotubes are "reversible," which means their size and orientation can be easily modified during the fabrication process. For the membrane, Xu and her collaborators used block copolymers - long sequences or "blocks" of one type of monomer molecule bound to blocks of another type of monomer molecule. Just as cyclic peptides self-assemble into nanotubes, block copolymers self-assemble into well-defined arrays of nanostructures over macroscopic distances. A polymer covalently linked to the cyclic peptide was used as a "mediator" to bind together these two self-assembling systems
"The polymer conjugate is the key," Xu says. "It controls the interface between the cyclic peptides and the block copolymers and synchronizes their self-assembly. The result is that nanotube channels only grow within the framework of the polymer membrane. When you can make everything work together this way, the process really becomes very simple."
Xu and her colleagues were able to fabricate subnanometer porous membranes measuring several centimeters across and featuring high-density arrays of channels. The channels were tested via gas transport measurements of carbon dioxide and neopentane. These tests confirmed that permeance was higher for the smaller carbon dioxide molecules than for the larger molecules of neopentane. The next step will be to use this technique to make thicker membranes.
"Theoretically, there are no size limitations for our technique so there should be no problem in making membranes over large area," Xu says. "We're excited because we believe this demonstrates the feasibility of synchronizing multiple self-assembly processes by tailoring secondary interactions between individual components. Our work opens a new avenue to achieving hierarchical structures in a multicomponent system simultaneously, which in turn should help overcome the bottleneck to achieving functional materials using a bottom-up approach."
This research was supported by DOE's Office of Science and by the U.S. Army Research Office. Measurements were carried out on beamlines at Berkeley Lab's Advanced Light Source and at the Advanced Photon Source of Argonne National Laboratory.
####
About Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory is a U.S. Department of Energy (DOE) national laboratory managed by the University of California for the DOE Office of Science. Berkeley Lab provides solutions to the world's most urgent scientific challenges including sustainable energy, climate change, human health, and a better understanding of matter and force in the universe. It is a world leader in improving our lives through team science, advanced computing, and innovative technology. Visit our at www.lbl.gov
For more information, please click here
Contacts:
Lynn Yarris
510-486-5375
Copyright © Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Thin films
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
![]() Understanding the mechanism of non-uniform formation of diamond film on tools: Paving the way to a dry process with less environmental impact March 24th, 2023
Understanding the mechanism of non-uniform formation of diamond film on tools: Paving the way to a dry process with less environmental impact March 24th, 2023
![]() New study introduces the best graphite films: The work by Distinguished Professor Feng Ding at UNIST has been published in the October 2022 issue of Nature Nanotechnology November 4th, 2022
New study introduces the best graphite films: The work by Distinguished Professor Feng Ding at UNIST has been published in the October 2022 issue of Nature Nanotechnology November 4th, 2022
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Self Assembly
![]() Liquid crystal templated chiral nanomaterials October 14th, 2022
Liquid crystal templated chiral nanomaterials October 14th, 2022
![]() Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
![]() Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
![]() Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanostructures get complex with electron equivalents: Nanoparticles of two different sizes break away from symmetrical designs January 14th, 2022
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Detection of bacteria and viruses with fluorescent nanotubes July 21st, 2023
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
Solar/Photovoltaic
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
Charged “molecular beasts” the basis for new compounds: Researchers at Leipzig University use “aggressive” fragments of molecular ions for chemical synthesis November 3rd, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








