Home > Nanotechnology Columns > UAlbany College of Nanoscale Science and Engineering > Novel Energy Storage Device: porous silicon ultracapacitor with superior performance
 |
Manisha V. Rane-Fondacaro CNSE Materials Scientist and Instructor UAlbany College of Nanoscale Science and Engineering |
Abstract:
Current large-scale battery and ultracapacitor technologies are limited in their capability, performance, durability, and cost since they do not use optimum materials, components, and integrated systems. Here at the Energy and Environmental Technology Applications Center (E2TAC), our approach offers tremendous potential as components for next generation batteries and ultracapacitors.
February 28th, 2011
Novel Energy Storage Device: porous silicon ultracapacitor with superior performance
Current large-scale battery and ultracapacitor technologies are limited in their capability, performance, durability, and cost since they do not use optimum materials, components, and integrated systems. Here at the Energy and Environmental Technology Applications Center (E2TAC), our approach offers tremendous potential as components for next generation batteries and ultracapacitors. For example, porous silicon electrodes created using nanostructures by design (with high surface area and high conductivity), are optimized in combination with compact-molecule ionic liquid electrolytes with large voltage windows and thermal stability. Together they result in devices with substantially improved equivalent series resistances, longer life, lower cost and overall breakthrough performance. In addition, the modular design enables mass manufacturability, and hence the potential for low cost systems below $200/kW creating a transformative impact for grid applications.
Energy storage in all its forms can enable large-scale penetration of renewable energy into the utility grid, reduce carbon emissions and nuclear waste from non-renewable energy sources, and improve the survivability and stability of the nation's utility grid. The electrochemical or double-layer capacitors devices will act as an important transient energy storage technology for rapid-charge/discharge applications, while batteries will provide longer-term storage solutions. Our goal includes enhancement of power density and energy density of battery and ultracapacitor based components; improvement in durability using new nano-materials and structures; increasing surface area of electrode, electrolyte interfaces; and identification of highly scalable nano-manufacturing approaches of the electrode/electrolyte assembly.
To achieve the goals indicated above, it is necessary to develop breakthrough components with lower costs and "order-of-magnitude" higher power and energy density for conventional batteries and ultracapacitors. The current technology uses activated carbon electrodes for many energy storage applications, which seriously lacks in performance for higher surface area materials. Moreover, the increase in pore volume is associated with reduction in pore wall thickness, which renders capacitance enhancement difficult. In case of nanoporous carbons, with periodic pore structure the charge storage mechanism is not restricted only to surface effects, but also amenable to intercalation. However, nanoporous carbons are (i) expensive by an order of magnitude than activated carbon material (ii) cannot be manufactured with tailored nanostructures by design or 3-D architectures or matched to meet the electrolytes characteristics of size, charge etc. Current supercapacitor technology is too expensive, costing about 0.5c/F ($1.28/kJ), while Li ion battery cost ~ $1090/kWh. There exists an urgent need for a paradigm shift in energy-storage device and component fabrication approaches, enabling cost competitiveness and performance enhancement, to realize the goal to conceptually develop and integrate all the components required for a large-scale (>10 MW) energy storage system. The novel technologies and methodologies we propose will facilitate the goal of creating a large-scale energy system that is not only cost effective, but also easily replicable and amenable to high volume manufacturing.
Addressing the problems with next generation technology enhancements:
Porous Silicon Electrodes:
We propose using porous silicon (p-Si) material to fabricate electrodes for electrochemical storage due to extreme flexibility of the material in terms of processing (easily tailorable microstructure), chemical inertness, as well as customizable surface area and conductivity. The conductivity is easily enhanced by plating with nickel and subsequent heat treatment to produce NiSi (resistivity-10.5 μΩ cm), which is lower by orders of magnitude than that of activated carbon (0.5-1.5 Ω cm), graphite ~ (2.6 x 104 Ω-1 cm-1). An inert material is especially helpful in enabling high voltage windows. Our electrodes are scalable and amenable to high volume manufacturing; thereby lowering the manufacturing cost based on economies of scale and increase reliability, and reproducibility of these components.
Optimization of Porous Silicon Based Electrodes:
Porous silicon based electrodes are created using partial electrochemical dissolution in hydrofluoric acid based solutions. Preliminary work in creating nanostructures in silicon using porous silicon and e-beam patterned electrodes comprised of uniform pore size structures via strict control over pore diameter and volume has shown considerable promise. A maximum capacitance of 320 μF/cm2 was achieved for porous silicon electrode system with an average diameter of 3 μm and pore depth up to 50 μm. Achieving porous silicon with sizes below 20 nm will increase surface area without diminishing power density. A surface area of 1000 m2/g would lead to a theoretical volumetric capacitance of 3200 F/g.
Why use Porous Silicon Electrodes for Electrochemical Storage?
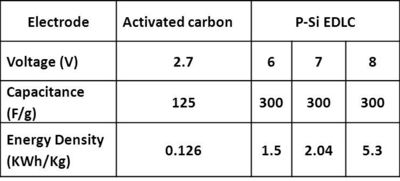 |
| Table 1: Comparison of p-Si with activated carbon electrode for capacitance and energy density |
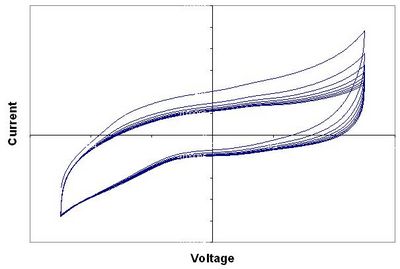 |
| Figure 1: CV of p-Si w/NiSi in 0.5 M H2SO4 |
A supercapacitor is a galvanic cell, where the aluminum (Al) current collector (CC) is in contact with activated carbon (AC). The current flows from AC into Al during operation, releases Al from the foil into the electrolyte during galvanic corrosion between Al and AC, various components of electrolyte, namely, fluorinated and heterocyclic compounds and hydrofluoric acid etc. attack the Al foil, thereby eventually destroying the surface oxide layer, followed by the formation of aluminum-oxy-fluoride species. Thus, the electrode proves to be Achilles heel of the device. We propose corrosion resistant porous silicon electrode as substitute for conventional activated carbon electrode.
The high surface area to volume ratio (> 500 m2/cc), simple fabrication technique, and fine tunable porosity (from μm-nm) makes it an ideal electrode candidate. The chemical inertness of p-Si enables exploiting entire voltage window (VW) offered by electrolyte. AC electrodes confine the VW to 2.7 V. We have tested p-Si in EDLC setting up to 4 V in 1.6 M TEABF4 / PC, and in 0.5M H2SO4 in our lab, and investigation with NiSi coating in high voltage window ionic liquid electrolytes developed in-house is underway. It is impossible to obtain monolithic electrodes with engineered porosity and conductivity using conventional carbon material. Electrodes based on p-Si material are easy to optimize for desired pore size, and conductivity due to simple fabrication and post fabrication steps.
Large Scale Manufacturing:
Fab manufactured p-Si electrodes enable superior design control and repeatability, in addition amenable to high volume and cost effective manufacturing. Use of porous-Si electrodes are reported for high efficiency fuel cells. A single cell yields power density ~140 mW/cm2, as compared with normal carbon based technology yields ~90 mW/cm2. Porous-Si technology is easily scalable, amenable to miniaturization, and conveniently transferrable for volume manufacturing at very low cost. We expect the life cycle $/Watt of p-Si based devices to be lower than the best activated carbon based devices, resulting from increasing mass manufacturability, ease of integration, long life arising from inert material, and rugged modules.
i. S. Desplobain, G. Gautier, J. Semai, L. Ventura and M. Roy, "Investigations on porous silicon as electrode material in electrochemical capacitors", Phys. Stat. Sol. (c), 4, 2180, 2007.
Ms. Rane-Fondacaro's Bio
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








