Home > Press > HKUST scientists discover new mechanisms of activity improvement on bimetallic catalysts for hydrogen generation and fuel cells
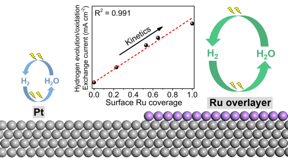 |
| Ruthenium atoms supported on platinum are extremely active to produce hydrogen CREDIT HKUST |
Abstract:
A group of researchers at the Hong Kong University of Science and Technology (HKUST) and Xiamen University has revealed new understandings of how surface ruthenium atoms can improve the hydrogen evolution and oxidation activities of platinum. This discovery opens a new venue for rational design of more advanced catalysts for electrolyzer and fuel cell applications.
HKUST scientists discover new mechanisms of activity improvement on bimetallic catalysts for hydrogen generation and fuel cells
Hong Kong, China | Posted on August 13th, 2021Hydrogen is a clean energy carrier that does not contain carbon. It is believed to play an essential role in our future sustainable society. Hydrogen can be produced from water via the hydrogen evolution reaction (HER) in an electrolyzer by using renewable energies, and consumed via a hydrogen oxidation reaction (HOR) in a fuel cell to generate electricity. Unfortunately, these two reactions are well-known kinetically sluggish in alkaline media, even on the most active platinum catalysts. The slow reaction rates limit the efficiencies of these two electrochemical devices and hinder their wide adoption. It has been known that the reaction rates of HER/HOR on platinum can be improved by surface modification or alloying with ruthenium. However, the mechanisms for this promotion have been under debate for over decades. Part of the reasons is a lack of direct observation of behaviors of hydrogen atoms on the surfaces of catalysts.
To reveal the enigma of high HER/HOR activities on platinum-ruthenium bimetallic catalysts, a research team led by Prof. Minhua Shao, Department of Chemical and Biological Engineering and Energy Institute at HKUST, recently applied the powerful surface-enhanced infrared absorption spectroscopy (SEIRAS) to directly monitor the binding strength of the important reaction intermediate, hydrogen atoms on various surfaces. Through the combined electrochemical, spectroscopic, and theoretical studies they confirmed the surface ruthenium atoms interacted with the sub-surface platinum is one order of magnitude more active than platinum, i.e., the ruthenium rather than platinum atoms are main active sites in this system.
“Previous works mainly used conventional electrochemical and characterization techniques, which cannot directly monitor the adsorption behavior of hydrogen reaction intermediates. In this work, we use the powerful surface-enhanced infrared absorption spectroscopy, which is among the very few techniques that can directly “see” surface hydrogen atoms, and provides us more straightforward information on how ruthenium improves the activity” said Prof. Shao. “This work rules out the most widespread theory that the bifunctional effect on the interface between platinum and ruthenium is the cause of increased activities, and opens new directions on future design of more advanced HER/HOR catalysts, which can consequently reduce the usage of precious metals in both water electrolyzers and hydrogen fuel cells.”
This work is part of the newly founded Collaborative Research Fund project led by Prof. Shao “Development of high-performance and long-life alkaline membrane fuel cells”, and constitutes an important subsection of fundamental research to this whole project. Following works on the development of practical and high-performance bimetallic platinum-ruthenium electrocatalysts based on these findings is in progress.
####
For more information, please click here
Contacts:
Johnny Tam
Office: 852-235-88556
Copyright © Hong Kong University of Science and Technology (HKUST)
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








