Home > Press > Carbon-loving materials designed to reduce industrial emissions
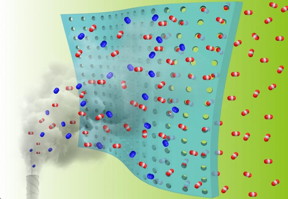 |
| Researchers at Oak Ridge National Laboratory and the University of Tennessee, Knoxville, demonstrated a novel fabrication method for affordable gas membranes that can remove carbon dioxide from industrial emissions. CREDIT Credit: Zhenzhen Yang/University of Tennessee |
Abstract:
Researchers at the Department of Energy's Oak Ridge National Laboratory and the University of Tennessee, Knoxville, are advancing gas membrane materials to expand practical technology options for reducing industrial carbon emissions.
Carbon-loving materials designed to reduce industrial emissions
Oak Ridge, TN | Posted on July 3rd, 2020Results published in Chem demonstrate a fabrication method for membrane materials that can overcome current bottlenecks in selectivity and permeability, key variables that drive carbon-capturing performance in real environments.
"Often there is a trade-off in how selective or how permeable you can make membranes that filter out carbon dioxide without allowing other gases to pass through. The ideal scenario is to create materials with high permeability and selectivity," said Zhenzhen Yang of UT's Department of Chemistry.
Gas membranes are a promising but still developing technology for reducing post-combustion or flue gas emissions produced by fossil-fueled industries.
The concept is simple: a thin, porous membrane acts as a filter for exhaust gas mixtures, selectively allowing carbon dioxide, or CO2, to flow through freely into a collector that is kept under reduced pressure, but preventing oxygen, nitrogen and other gases from tagging along.
Unlike existing chemical methods to capture CO2 from industrial processes, membranes are easy to install and can operate unattended for long periods with no additional steps or added energy costs. The catch is that new, cost-effective materials are needed to scale up the technology for commercial adoption.
"Gas membranes need pressure on one side and typically a vacuum on the other to maintain a free-flow environment, which is why materials' selectivity and permeability are so important to developing the technology," said Ilja Popovs of ORNL's Chemical Sciences Division. "Underperforming materials require more energy to push gases through the system, so advanced materials are key to keeping energy costs low."
No natural and only a few synthetic materials have exceeded what is called the Robeson upper limit, a known boundary that constrains how selective and permeable most materials can be before these rates start to drop.
Materials with sufficiently high selectivity and permeability for efficient gas separations are rare and often made from expensive starting materials whose production requires either long and tedious synthesis or costly transition metal catalysts.
"We set out to test a hypothesis that introducing fluorine atoms into membrane materials could improve carbon-capture and separation performance," Yang said.
The element fluorine, used to make consumer products such as Teflon and toothpaste, offers carbon dioxide-philic properties that make it attractive for carbon-capture applications. It is also widely available, making it a relatively affordable option for low-cost fabrication methods. Research on fluorinated gas membranes has been limited because of fundamental challenges of incorporating fluorine into materials to realize its carbon-loving functionality.
"Our first step was to create a unique fluorine-based polymer using simple chemical methods and commercially available starting materials," Yang said.
Next, researchers transformed, or carbonized, the material using heat to give it the porous structure and functionality needed for capturing CO2. The two-step process preserved the fluorinated groups and boosted CO2 selectivity in the final material, overcoming a fundamental hurdle encountered in other synthetic methods.
"The approach resulted in a carbon dioxide-philic material with high surface area and ultra-micropores that is stable in high-temperature operating conditions," Yang said. "All of these factors make it a promising candidate for carbon-capture and separation membranes."
The material's novel design contributes to its exceptional performance, observed in high selectivity and permeability rates that exceed the Robeson upper limit, something only a handful of materials have accomplished.
"Our success was a material achievement that demonstrates feasible routes for leveraging fluorine in future membrane materials. Moreover, we achieved this goal using commercially available, inexpensive starting materials," Popovs said.
The basic discovery expands the limited library of practical options for carbon-capture membranes and opens new directions for developing fluorinated membranes with other task-specific functionalities.
Researchers aim to next investigate the mechanism by which fluorinated membranes absorb and transport CO2, a fundamental step that will inform the design of better carbon-capture systems with materials purposely tailored to grab CO2 emissions.
####
About Oak Ridge National Laboratory
The research was supported by the DOE Office of Science.
UT-Battelle manages ORNL for the DOE Office of Science. The single largest supporter of basic research in the physical sciences in the United States, the Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit https://www.energy.gov/science .
For more information, please click here
Contacts:
Ashley Huff
865-241-6451
@ORNL
Copyright © Oak Ridge National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








