Home > Press > Fueling the World Sustainably: Synthesizing Ammonia using Less Energy
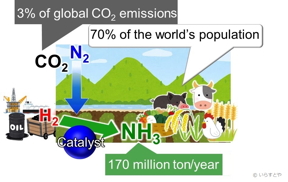 |
| Ammonia (NH3) is one of the most important industrial chemicals today, synthesized globally for use in fertilizers that then enable food production for approximately 70% of the world’s population. Ammonia is currently obtained by reacting nitrogen (N2) from air with hydrogen (H2). This reaction requires high energy and is, therefore, powered by fossil fuels, contributing to over 3% of the global CO2 emissions. |
Abstract:
Scientists at Tokyo Institute of Technology have developed an improved catalyst by taking the common dehydrating agent calcium hydride and adding fluoride to it. The catalyst facilitates the synthesis of ammonia at merely 50 °C, by using only half the energy that existing techniques require. This opens doors to ammonia production with low energy consumption and reduced greenhouse gas emission.
Fueling the World Sustainably: Synthesizing Ammonia using Less Energy
Tokyo, Japan | Posted on April 26th, 2020Ammonia is a critical for making plant fertilizer, which in turn feeds approximately 70% of the world’s population. In industries, ammonia is produced via the Haber-Bosch process, where methane is first reacted with steam to produce hydrogen, and hydrogen is then reacted with nitrogen to give ammonia. (Figure 1) The problem with this process is that as the temperature increases, the yield decreases. To continue to get a good yield, the pressure applied in the reaction chamber needs to be increased. This requires much energy. Further, the iron-based catalysts used for the reaction are only effective above 350 °C. Maintaining such high temperatures also requires a significant amount of energy. To top it all, the yield is only 30-40%.
Fossil fuels are currently used to power the process, contributing large amounts of carbon dioxide to the atmosphere. (Figure 1) Renewable resource alternatives, such as wind energy, have been applied, but those have not proven sustainable. To increase the yield while reducing harm to the environment, therefore, the reaction must take place at low temperatures. For this to happen, catalysts that enable the reaction at low temperatures are required.
So far, such catalysts have been elusive to scientists. “Conventional catalysts lose the catalytic activity for ammonia formation from N2 and H2 gases at 100-200 °C, even if they exhibit high catalytic performance at high temperatures,” remark a group of scientists from Tokyo Institute of Technology (Tokyo Tech), Japan, who appear to have finally solved the catalyst problem. The scientists, led by Dr. Michikazu Hara, developed a catalyst that is effective even at 50 °C. “Our catalyst produces ammonia from N2 and H2 gases at 50 °C with an extremely small activation energy of 20 kJ mol-1, which is less than half that reported for conventional catalysts,” Dr. Hara and colleagues report in their paper published in Nature Communications.
Their catalyst comprises a solid solution of CaFH, with ruthenium (Ru) nanoparticles deposited on its surface. The addition of fluoride (F-) to calcium hydride (CaH2), a common dehydrating agent, is what makes the catalyst effective at lower temperatures and pressures. After conducting spectroscopic and computational analyses, the scientists propose a possible mechanism by which the catalyst facilitates ammonia production.
The calcium–fluoride (Ca–F) bond is stronger than the calcium–hydrogen (Ca–H) bond. So, the presence of the Ca–F bond weakens the Ca–H bond and the Ru is able to extract H atoms from the catalyst crystal, leaving electrons in their place. The H atoms then desorb from the Ru nanoparticles as H2 gas. This occurs even at 50 °C. The resultant charge repulsion between the trapped electrons and F- ions in the crystal lower the energy barriers for these electrons to release, thereby giving the material high electron-donating capacity. These released electrons attack the bonds between the nitrogen atoms in the N2 gas, facilitating the production of ammonia (Figure 2).
This new method of ammonia production cuts energy demands, thereby reducing the carbon dioxide emissions from the use of large amounts of fossil fuels. The findings of this study illuminate the possibility of an environmentally sustainable Haber-Bosch process, opening the door to the next revolution in agricultural food production.
Reference
Authors:
Masashi Hattori1, Shinya Iijima1, Takuya Nakao2, Hideo Hosono2, Michikazu Hara1,*
Title of original paper:
Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C
Journal:
Nature Communications
DOI:
10.1038/s41467-020-15868-8
Affiliations:
1Laboratory for Materials and Structures, Tokyo Institute of Technology
2Materials Research Center for Element Strategy, Tokyo Institute of Technology
####
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
For more information, please click here
Contacts:
Emiko Kawaguchi
Public Relations group,
Tokyo Institute of Technology
+81-3-5734-2975
Copyright © Tokyo Institute of Technology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
Chemistry
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
![]() Quantum interference in molecule-surface collisions February 28th, 2025
Quantum interference in molecule-surface collisions February 28th, 2025
Videos/Movies
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Possible Futures
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Discoveries
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Food/Agriculture/Supplements
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() SMART researchers pioneer first-of-its-kind nanosensor for real-time iron detection in plants February 28th, 2025
SMART researchers pioneer first-of-its-kind nanosensor for real-time iron detection in plants February 28th, 2025
![]() Silver nanoparticles: guaranteeing antimicrobial safe-tea November 17th, 2023
Silver nanoparticles: guaranteeing antimicrobial safe-tea November 17th, 2023
Environment
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
![]() SMART researchers pioneer first-of-its-kind nanosensor for real-time iron detection in plants February 28th, 2025
SMART researchers pioneer first-of-its-kind nanosensor for real-time iron detection in plants February 28th, 2025
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








