Home > Press > What happens when you explode a chemical bond? Attosecond laser technique yields movies of chemical bond dissociation
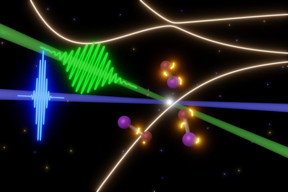 |
| UC Berkeley scientists are probing the fleeting steps in rapid photochemical reactions with some of the shortest laser pulses possible today. In this case, a femtosecond pulse of visible light (green) triggers the breakup of iodine monobromide molecules (center), while attosecond XUV laser pulses (blue) take snapshots of the molecules. This allows them to make a movie of the evolution of electronic states (yellow lights around molecules) before the molecules blow apart. CREDIT Yuki Kobayashi, UC Berkeley |
Abstract:
On bright summer days, the sunlight all around us is breaking bad by breaking bonds. Chemical bonds.
What happens when you explode a chemical bond? Attosecond laser technique yields movies of chemical bond dissociation
Berkeley, CA | Posted on July 12th, 2019Ultraviolet light shatters the links between atoms in the DNA of our skin cells, potentially causing cancer. UV light also breaks oxygen bonds, eventually creating ozone, and cleaves hydrogen off other molecules to leave behind free radicals that can damage tissue.
University of California, Berkeley, chemists using some of the shortest laser pulses available -- one quintillionth of a second -- have now been able to resolve the step-by-step process leading to the exploding of a chemical bond, essentially making a movie of the event. They can follow electrons indecisively bouncing around in various states in the molecule before the bond breaks, and the atoms go their separate ways.
The technique, reported last week in the journal Science, will help chemists understand and potentially manipulate chemical reactions stimulated by light, so-called photochemical reactions. Chemists and biologists, in particular, are interested in understanding how large molecules manage to absorb light energy without breaking any bonds, as happens when molecules in the eye absorb light, giving us vision, or molecules in plants absorb light for photosynthesis.
"Think about a molecule, rhodopsin, in the eye," said first author Yuki Kobayashi, a UC Berkeley doctoral student. "When light hits the retina, rhodopsin absorbs the visible light, and we can see things because rhodopsin's bond's conformation changes."
In fact, when the light energy is absorbed, a bond in rhodopsin twists, instead of breaks, triggering other reactions that result in the perception of light. The technique Kobayashi and his UC Berkeley colleagues, professors Stephen Leone and Daniel Neumark, developed could be used to study in detail how this light absorption leads to twisting after the molecule passes through an excited state called an avoided crossing or conical intersection.
To prevent the breaking of a bond in DNA, "you want to redirect the energy from dissociation to just being vibrationally hot. For rhodopsin, you want to redirect the energy from vibrating to a cis-trans isomerization, a twist," Kobayashi said. "These redirections of chemical reactions are happening ubiquitously around us, but we have not seen the actual moment of them before."
Fast laser pulses
Attosecond lasers -- an attosecond is a billionth of a billionth of a second -- have been around for about a decade and are used by scientists to probe very fast reactions. Since most chemical reactions occur rapidly, these fast-pulse lasers are key to "seeing" how the electrons that form the chemical bond behave when the bond breaks and/or reforms.
Leone, a professor of chemistry and of physics, is an experimentalist who also uses theoretical tools and is a pioneer in using attosecond lasers to probe chemical reactions. He has six of these X-ray and extreme ultraviolet (collectively, XUV) lasers in his UC Berkeley laboratory.
Working with one of the simplest of molecules, iodine monobromide (IBr) -- which is one iodine atom linked to one bromine atom -- the UC Berkeley team hit the molecules with an 8 femtosecond pulse of visible light to excite one of their outermost electrons, then probed them with attosecond laser pulses.
Pulsing the attosecond XUV laser at timed intervals of 1.5 femtosecond (a femtosecond is 1,000 attoseconds), much like using a strobe light, the researchers could detect the steps leading to the breakup of the molecules. The high-energy XUV laser was able to explore the excited energy states relative to the molecule's inner electrons, which normally do not participate in chemical reactions.
"You are kind of making a movie of the pathways of the electron when it approaches the crossing and the probability of it going along one path or along another," Leone said. "These tools we are developing allow you to look at solids, gases and liquids, but you need the shorter time scales (provided by an attosecond laser). Otherwise, you only see the beginning and the end, and you don't know what else happened in between."
The experiment showed clearly that the outer electrons of IBr, once excited, suddenly see a variety of states or places they could be and explore many of them before deciding which path to take. In this simple molecule, however, all paths lead to the electron settling on either iodine or bromine and the two atoms flying apart.
In larger molecules, which the team hopes soon to explore, excited electrons would have more choices, some where the energy goes into a twist, like with rhodopsin, or into molecular vibration without the molecules breaking apart.
"In biology, it turns out that evolution has selected things that are extremely effective at absorbing the energy and not breaking a bond," Leone said. "When something goes wrong in your chemistry is when you see diseases cropping up."
###
Other co-authors of the paper were Kristina Chang of UC Berkeley and Tao Zeng of Carleton University in Ottawa, Canada. Leone, the John R. Thomas Endowed Chair in Physical Chemistry, and Neumark, a UC Berkeley professor of chemistry, are also faculty scientists at Lawrence Berkeley National Laboratory.
####
For more information, please click here
Contacts:
Robert Sanders
510-643-6998
Copyright © University of California, Berkeley
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Cancer
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Military
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() New chip opens door to AI computing at light speed February 16th, 2024
New chip opens door to AI computing at light speed February 16th, 2024
Photonics/Optics/Lasers
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
![]() Optically trapped quantum droplets of light can bind together to form macroscopic complexes March 8th, 2024
Optically trapped quantum droplets of light can bind together to form macroscopic complexes March 8th, 2024
![]() HKUST researchers develop new integration technique for efficient coupling of III-V and silicon February 16th, 2024
HKUST researchers develop new integration technique for efficient coupling of III-V and silicon February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








