Home > Press > Defects help nanomaterial soak up more pollutant in less time: Rice U. researchers find new way to remove PFOS from industrial wastewater
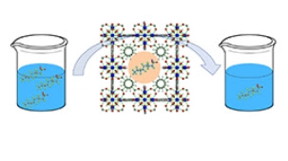 |
| By introducing defects into the structure of a metal-organic framework, or MOF, Rice University researchers found they could increase the amount of toxic pollutants called perfluorooctanesulfonic acid (PFOS) that the MOF could hold, as well as the speed with which it could adsorb them from heavily polluted industrial wastewater. (Image courtesy of Chelsea Clark/Rice University) |
Abstract:
Cleaning pollutants from water with a defective filter sounds like a non-starter, but a recent study by chemical engineers at Rice University found that the right-sized defects helped a molecular sieve soak up more perfluorooctanesulfonic acid (PFOS) in less time.
Defects help nanomaterial soak up more pollutant in less time: Rice U. researchers find new way to remove PFOS from industrial wastewater
Houston, TX | Posted on March 13th, 2019In a study in the American Chemical Society journal ACS Sustainable Chemistry and Engineering, Rice University researchers Michael Wong, Chelsea Clark and colleagues showed that a highly porous, Swiss cheese-like nanomaterial called a metal-organic framework (MOF) was faster at soaking up PFOS from polluted water, and that it could hold more PFOS, when additional nanometer-sized holes ("defects") were built into the MOF.
PFOS was used for decades in consumer products like stain-resistant fabrics and is the best-known member of a family of toxic chemicals called "per- and polyfluoroalkyl substances" (PFAS), which the Environmental Protection Agency (EPA) describes as "very persistent in the environment and in the human body -- meaning they don't break down and they can accumulate over time."
Wong, professor and chair of Rice's Department of Chemical and Biomolecular Engineering and a professor of chemistry, said, "We are taking a step in the right direction toward developing materials that can effectively treat industrial wastewaters in the parts-per-billion and parts-per-million level of total PFAS contamination, which is very difficult to do using current technologies like granular activated carbon or activated sludge-based systems."
Wong said MOFs, three-dimensional structures that self-assemble when metal ions interact with organic molecules called linkers, seemed like good candidates for PFAS remediation because they are highly porous and have been used to absorb and hold significant amounts of specific target molecules in previous applications. Some MOFs, for example, have a surface area larger than a football field per gram, and more than 20,000 kinds of MOFs are documented. In addition, chemists can tune MOF properties -- varying their structure, pore sizes and functions -- by tinkering with the synthesis, or chemical recipe that produces them.
Such was the case with Rice's PFAS sorbent. Clark, a graduate student in Wong's Catalysis and Nanomaterials Laboratory, began with a well-characterized MOF called UiO-66, and conducted dozens of experiments to see how various concentrations of hydrochloric acid changed the properties of the final product. She found she could introduce structural defects of various sizes with the method -- like making Swiss cheese with extra-big holes.
"The large-pore defects are essentially their own sites for PFOS adsorption via hydrophobic interactions," Clark said. "They improve the adsorption behavior by increasing the space for the PFOS molecules."
Clark tested variants of UiO-66 with different sizes and amounts of defects to determine which variety soaked up the most PFAS from heavily polluted water in the least amount of time.
"We believe that introducing random, large-pore defects while simultaneously maintaining the majority of the porous structure played a large role in improving the adsorption capacity of the MOF," she said. "This also maintained the fast adsorption kinetics, which is very important for wastewater remediation applications where contact times are short."
Wong said the study's focus on industrial concentrations of PFAS sets it apart from most previously published work, which has focused on cleaning polluted drinking water to meet the current federal standards of 70 parts per trillion. While treatment technologies like activated carbon and ion exchange resins can be effective for cleaning low-level concentrations of PFAS from drinking water, they are far less effective for treating high-concentration industrial waste.
Although PFAS use has been heavily restricted by international treaty since 2009, the chemicals are still used in semiconductor manufacturing and chrome plating, where wastewater can contain as much as one gram of PFAS per liter of water, or about 14 billion times the current EPA limit for safe drinking water.
"In general for carbon-based materials and ion-exchange resins, there is a trade-off between adsorption capacity and adsorption rate as you increase the pore size of the material," Wong said. "In other words, the more PFAS a material can soak up and trap, the longer it takes to fill up. In addition, carbon-based materials have been shown to be mostly ineffective at removing shorter-chain PFASs from wastewater.
"We found that our material combines high-capacity and fast-adsorption kinetics and also is effective for both long- and short-chain perfluoroalkyl sulfonates," he said.
Wong said it's difficult to beat carbon-based materials in terms of cost because activated carbon has been a mainstay for environmental filtration for decades.
"But it's possible if MOFs become produced on a large-enough scale," he said. "There are a few companies looking into commercial-scale production of UiO-66, which is one reason we chose to work with it in this study."
Additional co-authors include Kimberly Heck and Camilah Powell, both of Rice. The research was supported by the National Science Foundation (NSF) Graduate Research Fellowship Program and by the NSF's Nanosystems Engineering Research Center on Nanotechnology-Enabled Water Treatment (NEWT). Based at Rice, NEWT is a multi-institutional effort launched in 2015 to develop compact, mobile, off-grid water-treatment systems that can provide clean water to millions of people and make U.S. energy production more sustainable and cost-effective.
####
About Rice University
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 2 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Follow Rice News and Media Relations via Twitter @RiceUNews.
For more information, please click here
Contacts:
Jeff Falk
713-348-6775
Mike Williams
713-348-6728
Copyright © Rice University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Environment
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
Industrial
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
![]() Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
Nanotubes: a promising solution for advanced rubber cables with 60% less conductive filler June 1st, 2022
![]() Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
Protective equipment with graphene nanotubes meets the strictest ESD safety standards March 25th, 2022
![]() OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
OCSiAl receives the green light for Luxembourg graphene nanotube facility project to power the next generation of electric vehicles in Europe March 4th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








