Home > Press > Tripling the Energy Storage of Lithium-Ion Batteries: Scientists have synthesized a new cathode material from iron fluoride that surpasses the capacity limits of traditional lithium-ion batteries
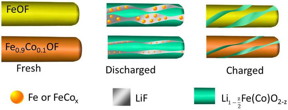 |
| Substituting the cathode material with oxygen and cobalt prevents lithium from breaking chemical bonds and preserves the material's structure. |
Abstract:
As the demand for smartphones, electric vehicles, and renewable energy continues to rise, scientists are searching for ways to improve lithium-ion batteries—the most common type of battery found in home electronics and a promising solution for grid-scale energy storage. Increasing the energy density of lithium-ion batteries could facilitate the development of advanced technologies with long-lasting batteries, as well as the widespread use of wind and solar energy. Now, researchers have made significant progress toward achieving that goal.
Tripling the Energy Storage of Lithium-Ion Batteries: Scientists have synthesized a new cathode material from iron fluoride that surpasses the capacity limits of traditional lithium-ion batteries
Upton, NY | Posted on June 14th, 2018A collaboration led by scientists at the University of Maryland (UMD), the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, and the U.S. Army Research Lab have developed and studied a new cathode material that could triple the energy density of lithium-ion battery electrodes. Their research was published on June 13 in Nature Communications.
"Lithium-ion batteries consist of an anode and a cathode," said Xiulin Fan, a scientist at UMD and one of the lead authors of the paper. "Compared to the large capacity of the commercial graphite anodes used in lithium-ion batteries, the capacity of the cathodes is far more limited. Cathode materials are always the bottleneck for further improving the energy density of lithium-ion batteries."
Scientists at UMD synthesized a new cathode material, a modified and engineered form of iron trifluoride (FeF3), which is composed of cost-effective and environmentally benign elements—iron and fluorine. Researchers have been interested in using chemical compounds like FeF3 in lithium-ion batteries because they offer inherently higher capacities than traditional cathode materials.
"The materials normally used in lithium-ion batteries are based on intercalation chemistry," said Enyuan Hu, a chemist at Brookhaven and one of the lead authors of the paper. "This type of chemical reaction is very efficient; however, it only transfers a single electron, so the cathode capacity is limited. Some compounds like FeF3 are capable of transferring multiple electrons through a more complex reaction mechanism, called a conversion reaction."
Despite FeF3's potential to increase cathode capacity, the compound has not historically worked well in lithium-ion batteries due to three complications with its conversion reaction: poor energy efficiency (hysteresis), a slow reaction rate, and side reactions that can cause poor cycling life. To overcome these challenges, the scientists added cobalt and oxygen atoms to FeF3 nanorods through a process called chemical substitution. This allowed the scientists to manipulate the reaction pathway and make it more "reversible."
"When lithium ions are inserted into FeF3, the material is converted to iron and lithium fluoride," said Sooyeon Hwang, a co-author of the paper and a scientist at Brookhaven's Center for Functional Nanomaterials (CFN). "However, the reaction is not fully reversible. After substituting with cobalt and oxygen, the main framework of the cathode material is better maintained and the reaction becomes more reversible."
To investigate the reaction pathway, the scientists conducted multiple experiments at CFN and the National Synchrotron Light Source II (NSLS-II)—two DOE Office of Science User Facilities at Brookhaven.
First at CFN, the researchers used a powerful beam of electrons to look at the FeF3 nanorods at a resolution of 0.1 nanometers—a technique called transmission electron microscopy (TEM). The TEM experiment enabled the researchers to determine the exact size of the nanoparticles in the cathode structure and analyze how the structure changed between different phases of the charge-discharge process. They saw a faster reaction speed for the substituted nanorods.
"TEM is a powerful tool for characterizing materials at very small length scales, and it is also able to investigate the reaction process in real time," said Dong Su, a scientist at CFN and a co-corresponding author of the study. "However, we can only see a very limited area of the sample using TEM. We needed to rely on the synchrotron techniques at NSLS-II to understand how the whole battery functions."
At NSLS-II's X-ray Powder Diffraction (XPD) beamline, scientists directed ultra-bright x-rays through the cathode material. By analyzing how the light scattered, the scientists could "see" additional information about the material's structure.
"At XPD, we conducted pair distribution function (PDF) measurements, which are capable of detecting local iron orderings over a large volume," said Jianming Bai, a co-author of the paper and a scientist at NSLS-II. "The PDF analysis on the discharged cathodes clearly revealed that the chemical substitution promotes electrochemical reversibility."
Combining highly advanced imaging and microscopy techniques at CFN and NSLS-II was a critical step for assessing the functionality of the cathode material.
"We also performed advanced computational approaches based on density functional theory to decipher the reaction mechanism at an atomic scale," said Xiao Ji, a scientist at UMD and co-author of the paper. "This approach revealed that chemical substitution shifted the reaction to a highly reversible state by reducing the particle size of iron and stabilizing the rocksalt phase."Scientists at UMD say this research strategy could be applied to other high energy conversion materials, and future studies may use the approach to improve other battery systems.
This study was supported by the U.S. Army Research Laboratory and DOE's Office of Energy Efficiency and Renewable Energy. Operations at CFN and NSLS-II are supported by DOE's Office of Science
####
About Brookhaven National Laboratory
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Follow @BrookhavenLab on Twitter or find us on Facebook.
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE's Office of Science by Brookhaven Science Associates, a limited-liability company founded by the Research Foundation for the State University of New York on behalf of Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit applied science and technology organization.
For more information, please click here
Contacts:
Stephanie Kossman
(631) 344-8671
or Peter Genzer
, (631) 344-3174
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Military
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() New chip opens door to AI computing at light speed February 16th, 2024
New chip opens door to AI computing at light speed February 16th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








