Home > Press > 'Helix-to-Tube,' a simple strategy to synthesize covalent organic nanotubes: Making mechanically strong nanotubes with light
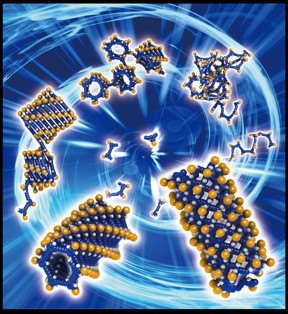 |
| Synthesis of organic nanotubes by the "helix-to-tube" method. CREDIT: ITbM, Nagoya University |
Abstract:
Kaho Maeda, Dr. Hideto Ito, Professor Kenichiro Itami of the JST-ERATO Itami Molecular Nanocarbon Project and the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University, and their colleagues have reported in the Journal of the American Chemical Society, on the development of a new and simple strategy, "helix-to-tube" to synthesize covalent organic nanotubes.
'Helix-to-Tube,' a simple strategy to synthesize covalent organic nanotubes: Making mechanically strong nanotubes with light
Nagoya, Japan | Posted on September 1st, 2016Organic nanotubes (ONTs) are organic molecules with tubular nanostructures. Nanostructures are structures that range between 1 nm and 100 nm, and ONTs have a nanometer-sized cavity. Various applications of ONTs have been reported, including molecular recognition materials, transmembrane ion channel/sensors, electro-conductive materials, and organic photovoltaics. Most ONTs are constructed by a self-assembly process based on weak non-covalent interactions such as hydrogen bonding, hydrophobic interactions and π-π interactions between aromatic rings. Due to these relatively weak interactions, most non-covalent ONTs possess a relatively fragile structure.
Covalent ONTs, whose tubular skeletons are cross-linked by covalent bonding (a bond made by sharing of electrons between atoms) could be synthesized from non-covalent ONTs. While covalent ONTs show higher stability and mechanical strength than non-covalent ONTs, the general synthetic strategy for covalent ONTs was yet to be established.
A team led by Hideto Ito and Kenichiro Itami has succeeded in developing a simple and effective method for the synthesis of robust covalent ONTs (tube) by an operationally simple light irradiation of a readily accessible helical polymer (helix). This so-called "helix-to-tube" strategy is based on the following steps: 1) polymerization of a small molecule (monomer) to make a helical polymer followed by, 2) light-induced cross-linking at longitudinally repeating pitches across the whole helix to form covalent nanotubes.
With their strategy, the team designed and synthesized diacetylene-based helical polymers (acetylenes are molecules that contain carbon-carbon triple bonds), poly(m-phenylene diethynylene)s (poly-PDEs), which has chiral amide side chains that are able to induce a helical folding through hydrogen-bonding interactions.
The researchers revealed that light-induced cross-linking at longitudinally aligned 1,3-butadiyne moieties (a group of molecules that contain four carbons with triple bonds at the first and third carbons) could generate the desired covalent ONT. "This is the first time in the world to show that the photochemical polymerization reaction of diynes is applicable to the cross-linking reaction of a helical polymer," says Maeda, a graduate student who mainly conducted the experiments.
The "helix-to-tube" method is expected to be able to generate a range of ONT-based materials by simply changing the arene (aromatic ring) unit in the monomer.
"One of the most difficult parts of this research was how to obtain scientific evidence on the structures of poly-PDEs and covalent ONTs," says Ito, one of the leaders of this study. "We had little experience with the analysis of polymers and macromolecules such as ONTs. Fortunately, thanks to the support of our collaborators in Nagoya University, who are specialists in these particular research fields, we finally succeeded in characterizing these macromolecules by various techniques including spectroscopy, X-ray diffraction, and microscopy."
"Although it took us about a year to synthesize the covalent ONT, it took another one and a half year to determine the structure of the nanotube," says Maeda. "I was extremely excited when I first saw the transmission electron microscopy (TEM) images, which indicated that we had actually made the covalent ONT that we were expecting," she continues.
"The best part of the research for me was finding that the photochemical cross-linking had taken place on the helix for the first time," says Maeda. "In addition, photochemical cross-linking is known to usually occur in the solid phase, but we were able to show that the reaction takes place in the solution phase as well. As the reactions have never been carried out before, I was dubious at first, but it was a wonderful feeling to succeed in making the reaction work for the first time in the world. I can say for sure that this was a moment where I really found research interesting."
"We were really excited to develop this simple yet powerful method to achieve the synthesis of covalent ONTs," says Itami, the director of the JST-ERATO project and the center director of ITbM. "The "helix-to-tube" method enables molecular level design and will lead to the synthesis of various covalent ONTs with fixed diameters and tube lengths with desirable functionalities."
"We envisage that ongoing advances in the "helix-to-tube" method may lead to the development of various ONT-based materials including electro-conductive materials and luminescent materials," says Ito. "We are currently carrying out work on the "helix-to-tube" methodology and we hope to synthesize covalent ONTs with interesting properties for various applications."
####
About Nagoya University
JST-ERATO Itami Molecular Nanocarbon Project The JST-ERATO Itami Molecular Nanocarbon Project was launched at Nagoya University in April 2014. This is a 5-year project that seeks to open the new field of nanocarbon science. This project entails the design and synthesis of as-yet largely unexplored nanocarbons as structurally well-defined molecules, and the development of novel, highly functional materials based on these nanocarbons. Researchers combine chemical and physical methods to achieve the controlled synthesis of well-defined uniquely structured nanocarbon materials, and conduct interdisciplinary research encompassing the control of molecular arrangement and orientation, structural and functional analysis, and applications in devices and biology. The goal of this project is to design, synthesize, utilize, and understand nanocarbons as molecules.
About WPI-ITbM
The Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. ITbM is one of the research centers of the Japanese MEXT (Ministry of Education, Culture, Sports, Science and Technology) program, the World Premier International Research Center Initiative (WPI). The aim of ITbM is to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a "Mix-Lab" style, where international young researchers from various fields work together side-by-side in the same lab, enabling interdisciplinary interaction. Through these endeavors, ITbM will create "transformative bio-molecules" that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
About JST-ERATO
ERATO (The Exploratory Research for Advanced Technology), one of the Strategic Basic Research Programs, aims to form a headstream of science and technology, and ultimately contribute to science, technology, and innovation that will change society and the economy in the future. In ERATO, a Research Director, a principal investigator of ERATO research project, establishes a new research base in Japan and recruits young researchers to implement his or her challenging research project within a limited time frame.
For more information, please click here
Contacts:
Author Contact
Professor Kenichiro Itami
JST-ERATO Itami Molecular Nanocarbon Project
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-788-6098
Dr. Hideto Ito
Graduate School of Science, Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-3551 FAX: +81-52-788-6098
Media Contact
Dr. Aki Miura
JST-ERATO Itami Molecular Nanocarbon Project, Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-789-5916
Dr. Ayako Miyazaki
81-527-894-999
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
Copyright © Nagoya University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings/Nanosheets
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Photonics/Optics/Lasers
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








