Home > Press > Nanobiotix reports successful results from Phase I/II Trial of NBTXR3 in Head & Neck Cancer
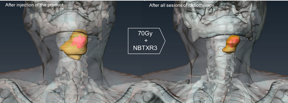 |
| Figure: Patient treated with NBTXR3; 3D scan reconstruction before and after radiotherapy, showing tumor reduction and presence of the nanoparticles in the tumor with no leakage in surrounding healthy tissues. (In yellow Tumor. In pink NBTXR3). |
Abstract:
Primary safety and feasibility endpoints achieved
Preliminary positive signs of antitumoral effect in all evaluable patients
Company preparing now registration clinical plan, including EU and USA
Nanobiotix reports successful results from Phase I/II Trial of NBTXR3 in Head & Neck Cancer
Paris, France, Cambridge, MA | Posted on July 6th, 2016NANOBIOTIX (Euronext: NANO – ISIN: FR0011341205), a late clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer, today announced positive results from a Phase I/II clinical trial of its lead product, NBTXR3, for the treatment of locally advanced cancers of the oral cavity, tongue or oropharynx (head and neck cancer, or H&N) in frail and elderly patients.
Nanobiotix’s lead product, NBTXR3, is a first-in-class nanoparticle radio-enhancer designed for direct injection into cancerous tumors and engineered to increase the dose and efficacy of radiotherapy without increasing toxicity or causing damage to surrounding healthy tissues. The intended use of NBTXR3 in this head and neck cancer patient population is to improve current radiotherapy outcomes by achieving better local control of the tumor and improving systemic benefit as well as quality of life (QoL).
The prospective, open-label, non-randomized, multicentre, dose escalation Phase I/II trial met its primary endpoint of safety and tolerability of NBTXR3. Ten Head and Neck cancer patients have been treated for the first 3 dose levels (out of 4). A Data Safety Monitoring Board (DSMB) composed by external experts, has confirmed the excellent safety profile, with no related serious adverse events, the feasibility of the injection and appropriate distribution.
The study showed promising signs of tumor volume response in a cancer patient population with a high unmet medical need, that cannot receive the standard of care (radiotherapy plus chemotherapy). 7 out 7 evaluable patients had a response with a tumor volume reduction equal or superior to 50% (outside 2 non evaluable, patient number 10 evaluation on going).
Based on these promising results, Nanobiotix is currently establishing a clinical development plan, potentially in EU and USA, which could lead to the registration of NBTXR3 for use in this indication.
Elsa Borghi, MD, CMO of Nanobiotix, commented: “The results seen in this study are exciting. Frail and elderly head and neck cancer patients, have few therapeutic options. Our findings show that NBTXR3 has a very good safety profile and promising tumor reduction, which could make a valuable difference for these patients. We are now working to prepare the next clinical trial.”
Laurent Levy, CEO of Nanobiotix commented: “These results represent major advances in the global clinical development of NBTXR3. We have now observed homogeneity of comparable Phase I/II results between soft tissue sarcoma and head and neck cancer, two very different oncology indications. At this step, the behavior and effect of the product are similar across these studies. This brings us a significant step closer towards proving the transferability of our approach, from one type of tumor to the other. We are enthusiastic about the increased likelihood of being able to use NBTXR3 to treat a large number of patients across cancer types.”
Clinical trial: 1. Design, 2. Data and 3. Next steps for development
NBTXR3 phase I/II trial in Head & Neck cancer
A significant proportion of head and neck carcinomas in the western world are found in the oral cavity, and the oropharynx, the posterior continuation of the oral cavity that connects with the nasopharynx (above) and laryngopharynx (below).
These structures play a crucial role in swallowing, breathing and speaking. Locally advanced oropharyngeal cancers can obstruct the airflow or infiltrate muscles or nerves, significantly disrupting essential local functions. Response in H&N cancer patients is related to: Age, stage, size, comorbidity, localization of the tumor and infection by the human papilloma virus (presence versus absence of HPV).
Local control of the tumor, when possible, is critical to preserve organ function, quality of life and has a direct impact on the disease outcome including Progression – Free Survival and (PFS) Overall Survival (OS).
1. Design
The target population for the Phase I/II trial are patients with locally advanced squamous cell carcinoma of the oral cavity, tongue or oropharynx (Stage T3 and T4), who are also classified as frail and elderly. They have a poorer prognosis as compared to other H&N cancer patients. In this population tumor response and local control are usually very low compare to patients eligible for combined treatment: radiotherapy plus cisplatin.
This study has targeted patients with bulky tumors, with significant invasion of local tissues. In order to ensure the optimal treatment for every patient, the design of the study has included two routes of injection of NBTXR3: intratumoral injection and super selective intra-arterial injection.
Arm 1: Intra Tumoral (IT) injection, Dose escalation (5%, 10%, 15%, 22% of the tumor volume). Number of patients could go up to 20 (3 to 6 patients per dose level could be treated; 3 if no safety issues).
Arm 2: Intra Arterial (IA) injection, Dose escalation (5%, 10%, 15%, 22% of the tumor volume). Number of patients could go up to 20 (3 to 6 patients per dose level could be treated; 3 if no safety issues).
Patients received 35 daily sessions (2GY per session) of radiotherapy starting one day after the injection of NBTXR3 with a total of 70Gy (standard of care).
At 50Gy (71% of the total dose) tumor volume is evaluated to assess the possibility of the patient to continue RTx (if tumor volume shrinkage is more than 50%) and avoid further unnecessary radiation toxicity and salvage surgery.
2. Data
2.1 Primary Endpoint: very good Safety profile observed
Evaluation confirm NBTXR3 has a very good safety profile in this patient population. No Adverse Events (AEs) related to NBTXR3 have been observed.
The observed adverse events were all related to either radiation therapy or the disease itself.
All the patients treated so far have completed their radiation therapy, confirming very good local tolerability profile of the product.
Numerous Adverse events have been observed in the study, which occurrence by level and causality assessment are presented following in table.
Demonstrated feasibility and appropriate distribution of the product
The selection of the route of administration is determined by the tumor size and mainly by the topography and shape. So far, the study has demonstrated that despite of the heterogeneity of tumors, the intratumoral injection is feasible and very well tolerated. This is a positive finding because the intra-tumoral injection is a shorter, simpler procedure, than the super selective intra-arterial injection. Moreover, it can be easily included in the medical practice.
Arm 2 has not been explored as the IT injection in arm 1 has been shown to be feasible and successful.
The feasibility of NBTXR3 injection at the first three dose levels (5%, 10% and 15% of the tumor volume) have been validated by the Data Safety Monitoring Board (DSMB), a safety committee of experts.
Moreover, the product appears to stay within the tumor with no leakage in the surrounding healthy tissues from the day of injection until the end of radiotherapy treatment.
The recruitment at the fourth and last dose level of 22% is ongoing.
2.2 Secondary endpoint: Tumor Response
Secondary endpoints of this trial include the assessment by MRI of the overall response, the evaluation of local progression-free survival (LPFS) and PFS.
Tumor Response has been measured during the radiotherapy treatment after 50GY (71% of the total dose delivered to patient) and at the end of the treatment after 70Gy (100% of the total dose delivered to patient) by imaging using MRI.
The response is established based on tumor volume and decrease of the longest dimension (RECIST 1.1 criteria).
Promising signs of efficacy has been reported with 7/7 patients showing tumor volume response superior or equal to 50%. 2 patients have shown complete or near complete tumor volume shrinkage.
In the follow up of this trial, Nanobiotix is monitoring LPFS, PFS and OS for all patients.
3. Next Steps for Development
A second trial has been planned that will include NBTXR3 in combination with standard of care (SOC) treatment, cisplatin plus radiotherapy. Currently, approximately 35-40% of patients with head and neck carcinomas receive treatment of cisplatin with radiotherapy. Treating this group with NBTXR3 would magnify the total potential treatable population in this indication.
Nanobiotix is currently establishing the clinical development plan, which could lead to the registration of NBTXR3 for use in this Head and neck patient.
In addition to H&N cancer, NBTXR3 is currently under clinical development for soft tissue sarcoma (registration phase), prostate cancer, rectal cancer (PharmaEngine) and liver cancers (HCC and liver metastases).
####
About Nanobiotix
Nanobiotix (Euronext: NANO / ISIN: FR0011341205) is a late clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer. The Company’s first-in-class, proprietary technology, NanoXray, enhances radiotherapy energy with a view to provide a new, more efficient treatment for cancer patients.
NanoXray products are compatible with current radiotherapy treatments and are meant to treat potentially a wide variety of solid tumors including soft tissue sarcoma, head and neck cancers, liver cancers, prostate cancer, breast cancer, glioblastoma, etc., via multiple routes of administration.
Nanobiotix’s lead product NBTXR3, based on NanoXray, is currently under clinical development for soft tissue sarcoma, head and neck cancer, prostate cancer, rectal cancer (PharmaEngine) and liver cancers (HCC and liver metastases). The Company has partnered with PharmaEngine for clinical development and commercialization of NBTXR3 in Asia.
Nanobiotix is listed on the regulated market of Euronext in Paris (ISIN: FR0011341205, Euronext ticker: NANO, Bloomberg: NANO: FP). The Company Headquarter is based in Paris, France. Affiliate in Cambridge, United States.
For more information, please visit www.nanobiotix.com
Issued for and on behalf of Nanobiotix by Instinctif Partners.
Disclaimer
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the reference document of Nanobiotix registered by the French Financial Markets Authority (Autorité des marchés financiers) on January 12, 2016 under number R.16-001 (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country.
For more information, please click here
Contacts:
Sarah Gaubert
Head of Communication and Public Affairs
+33 (0)1 40 26 07 55
Media relations
France - NewCap
Annie-Florence Loyer
+33 (0)6 88 20 35 59
EU Outside France - Instinctif Partners
Melanie Toyne Sewell
+44 (0) 207 457 2020
United States - The Ruth Group
Kirsten Thomas / Chris Hippolyte
+1 508-280-6592 / +1 646-536-7023
Copyright © Nanobiotix
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








