Home > Press > Speedy ion conduction in solid electrolytes clears road for advanced energy devices
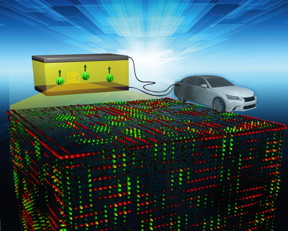 |
| An ORNL-led research team found the key to fast ion conduction in a solid electrolyte. Tiny features maximize ion transport pathways, represented by red and green. CREDIT: Oak Ridge National Laboratory, U.S. Dept. of Energy |
Abstract:
In a rechargeable battery, the electrolyte transports lithium ions from the negative to the positive electrode during discharging. The path of ionic flow reverses during recharging. The organic liquid electrolytes in commercial lithium-ion batteries are flammable and subject to leakage, making their large-scale application potentially problematic. Solid electrolytes, in contrast, overcome these challenges, but their ionic conductivity is typically low.
Speedy ion conduction in solid electrolytes clears road for advanced energy devices
Oak Ridge, TN | Posted on May 5th, 2016Now, a team led by the Department of Energy's Oak Ridge National Laboratory has used state-of-the-art microscopy to identify a previously undetected feature, about 5 billionths of a meter (nanometers) wide, in a solid electrolyte. The work experimentally verifies the importance of that feature to fast ion transport, and corroborates the observations with theory. The new mechanism the researchers report in Advanced Energy Materials points out a new strategy for the design of highly conductive solid electrolytes.
"The solid electrolyte is one of the most important factors in enabling safe, high-power, high-energy, solid-state batteries," said first author Cheng Ma of ORNL, who conducted most of the study's experiments. "But currently the low conductivity has limited its applications."
ORNL's Miaofang Chi, the senior author, said, "Our work is basic science focused on how we can facilitate ion transport in solids. It is important to the design of fast ion conductors, not only for batteries, but also for other energy devices." These include supercapacitors and fuel cells.
To directly observe the atomic arrangement in the solid electrolyte, the researchers used aberration-corrected scanning transmission electron microscopy to send electrons through a sample. To observe an extremely small feature in a three-dimensional (3D) material with a method that essentially provides a two-dimensional (2D) projection, they needed a sample of extraordinary thinness. To prepare one, they relied on comprehensive materials processing and characterization capabilities of the Center for Nanophase Materials Sciences, a DOE Office of Science User Facility at ORNL.
"Usually the transmission electron microscopy specimen is 20 nanometers thick, but Ma developed a method to make the specimen ultra-thin (approximately 5 nanometers)," Chi said. "That was the key because such a thickness is comparable to the size of the hidden feature we finally resolved."
The researchers examined a prototype system called LLTO, shorthand for its lithium, lanthanum, titanium and oxygen building blocks. LLTO possesses the highest bulk conductivity among oxide systems.
In this material, lithium ions move fastest in the planar 2D pathways resulting from alternating stacks of atomic layers rich in either lanthanum or lithium. The ORNL-led team was the first to see that, without hurting this superior 2D transport, tiny domains, or fine features approximately 5 to 10 nanometers wide, throughout the 3D material provided more directions in which lithium ions could move. The domains looked like sets of shelves stacked at right angles to others. The smaller the shelves, the easier it was for ions to flow in the direction of an applied current.
ORNL's Yongqiang Cheng and Bobby Sumpter performed molecular dynamics simulations that corroborated the experimental findings.
Previously, scientists looked at the atomic structure of the simplest repeating unit of a crystal--called a unit cell--and rearranged its atoms or introduced different elements to see how they could facilitate ion transport. Unit cells are typically less than 1 nanometer wide. In the material that the ORNL scientists studied for this paper, the unit cell is nearly half a nanometer. The team's unexpected finding--that fine features, of only a few nanometers and traversing a few unit cells, can maximize the number of ionic transport pathways--provides new perspective.
"The finding adds a new criterion," Chi said. "This largely overlooked length scale could be the key to fast ionic conduction."
Researchers will need to consider phenomena on the order of several nanometers when designing materials for fast ion conduction.
Ma agreed. "The prototype material has high ionic conductivity because not only does it maintain unit-cell structure, but also it adds this fine feature, which underpins 3D pathways," Ma said. "We're not saying that we shouldn't be looking at the unit-cell scale. We're saying that in addition to the unit cell scale, we should also be looking at the scale of several unit cells. Sometimes that outweighs the importance of one unit cell."
For several decades, when researchers had no explanation for certain material behaviors, they speculated phenomena transcending one unit cell could be at play. But they never saw evidence. "This is the first time we proved it experimentally," Ma said. "This is a direct observation, so it is the most solid evidence."
The title of the paper is "Mesoscopic Framework Enables Facile Ionic Transport in Solid Electrolytes for Li-ion Batteries."
###
The DOE Office of Science supported electron microscopy, theory calculations and electrochemical analysis. Work was performed at the Center for Nanophase Materials Sciences, a DOE Office of Science User Facility at ORNL. Researchers at Tsinghua University in China participated in materials synthesis and electrochemical analysis.
####
About Oak Ridge National Laboratory
UT-Battelle manages ORNL for DOE's Office of Science. The single largest supporter of basic research in the physical sciences in the United States, the Office of Science is working to address some of the most pressing challenges of our time. For more information, please visit www.science.energy.gov
For more information, please click here
Contacts:
Dawn Levy
865-576-6448
Copyright © Oak Ridge National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








