Home > Press > Relieving electric vehicle range anxiety with improved batteries: Lithium-sulfur batteries last longer with nanomaterial-packed cathode
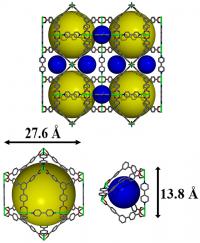 |
| Pacific Northwest National Laboratory developed a nickel-based metal organic framework, shown here in an illustration, to hold onto polysulfide molecules in the cathodes of lithium-sulfur batteries and extend the batteries' lifespans. The colored spheres in this image represent the 3D material's tiny pores into with the polysulfides become trapped.
Credit: Pacific Northwest National Laboratory |
Abstract:
Electric vehicles could travel farther and more renewable energy could be stored with lithium-sulfur batteries that use a unique powdery nanomaterial.
Relieving electric vehicle range anxiety with improved batteries: Lithium-sulfur batteries last longer with nanomaterial-packed cathode
Richland, WA | Posted on April 16th, 2014Researchers added the powder, a kind of nanomaterial called a metal organic framework, to the battery's cathode to capture problematic polysulfides that usually cause lithium-sulfur batteries to fail after a few charges. A paper describing the material and its performance was published online April 4 in the American Chemical Society journal Nano Letters.
"Lithium-sulfur batteries have the potential to power tomorrow's electric vehicles, but they need to last longer after each charge and be able to be repeatedly recharged," said materials chemist Jie Xiao of the Department of Energy's Pacific Northwest National Laboratory. "Our metal organic framework may offer a new way to make that happen."
Today's electric vehicles are typically powered by lithium-ion batteries. But the chemistry of lithium-ion batteries limits how much energy they can store. As a result, electric vehicle drivers are often anxious about how far they can go before needing to charge. One promising solution is the lithium-sulfur battery, which can hold as much as four times more energy per mass than lithium-ion batteries. This would enable electric vehicles to drive farther on a single charge, as well as help store more renewable energy. The down side of lithium-sulfur batteries, however, is they have a much shorter lifespan because they can't currently be charged as many times as lithium-ion batteries.
Energy Storage 101
The reason can be found in how batteries work. Most batteries have two electrodes: one is positively charged and called a cathode, while the second is negative and called an anode. Electricity is generated when electrons flow through a wire that connects the two. To control the electrons, positively charged atoms shuffle from one electrode to the other through another path: the electrolyte solution in which the electrodes sit.
The lithium-sulfur battery's main obstacles are unwanted side reactions that cut the battery's life short. The undesirable action starts on the battery's sulfur-containing cathode, which slowly disintegrates and forms molecules called polysulfides that dissolve into the liquid electrolyte. Some of the sulfur — an essential part of the battery's chemical reactions — never returns to the cathode. As a result, the cathode has less material to keep the reactions going and the battery quickly dies.
New materials for better batteries
Researchers worldwide are trying to improve materials for each battery component to increase the lifespan and mainstream use of lithium-sulfur batteries. For this research, Xiao and her colleagues honed in on the cathode to stop polysulfides from moving through the electrolyte.
Many materials with tiny holes have been examined to physically trap polysulfides inside the cathode. Metal organic frameworks are porous, but the added strength of PNNL's material is its ability to strongly attract the polysulfide molecules.
The framework's positively charged nickel center tightly binds the polysulfide molecules to the cathodes. The result is a coordinate covalent bond that, when combined with the framework's porous structure, causes the polysulfides to stay put.
"The MOF's highly porous structure is a plus that further holds the polysulfide tight and makes it stay within the cathode," said PNNL electrochemist Jianming Zheng.
Nanomaterial is key
Metal organic frameworks — also called MOFs — are crystal-like compounds made of metal clusters connected to organic molecules, or linkers. Together, the clusters and linkers assemble into porous 3-D structures. MOFs can contain a number of different elements. PNNL researchers chose the transition metal nickel as the central element for this particular MOF because of its strong ability to interact with sulfur.
During lab tests, a lithium-sulfur battery with PNNL's MOF cathode maintained 89 percent of its initial power capacity after 100 charge-and discharge cycles. Having shown the effectiveness of their MOF cathode, PNNL researchers now plan to further improve the cathode's mixture of materials so it can hold more energy. The team also needs to develop a larger prototype and test it for longer periods of time to evaluate the cathode's performance for real-world, large-scale applications.
PNNL is also using MOFs in energy-efficient adsorption chillers and to develop new catalysts to speed up chemical reactions.
"MOFs are probably best known for capturing gases such as carbon dioxide," Xiao said. "This study opens up lithium-sulfur batteries as a new and promising field for the nanomaterial."
This research was funded by the Department of Energy's Office of Energy Efficiency and Renewable Energy. Researchers analyzed chemical interactions on the MOF cathode with instruments at EMSL, DOE's Environmental Molecular Sciences Laboratory at PNNL.
In January, a Nature Communications paper by Xiao and some of her PNNL colleagues described another possible solution for lithium-sulfur batteries: developing a hybrid anode that uses a graphite shield to block polysulfides.
###
Reference: Jianming Zheng, Jian Tian, Dangxin Wu, Meng Gu, Wu Xu, Chongmin Wang, Fei Gao, Mark H. Engelhard, Ji-Guang Zhang, Jun Liu & Jie Xiao, "Lewis Acid-Base Interactions Between Polysulfides and Metal Organic Framework in Lithium Sulfur Batteries," Nano Letters, published online April 4, 2014, DOI: 10.1021/nl404721h.
####
About DOE/Pacific Northwest National Laboratory
Interdisciplinary teams at Pacific Northwest National Laboratory address many of America's most pressing issues in energy, the environment and national security through advances in basic and applied science. Founded in 1965, PNNL employs 4,300 staff and has an annual budget of about $950 million. It is managed by Battelle for the U.S. Department of Energy. For more information, visit the PNNL News Center, or follow PNNL on Facebook, Google+, LinkedIn and Twitter.
EMSL, the Environmental Molecular Sciences Laboratory, is a national scientific user facility sponsored by the Department of Energy's Office of Science. Located at Pacific Northwest National Laboratory in Richland, Wash., EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. Its integrated computational and experimental resources enable researchers to realize important scientific insights and create new technologies. Follow EMSL on Facebook, LinkedIn and Twitter.
For more information, please click here
Contacts:
Franny White
509-375-6904
Copyright © DOE/Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Laboratories
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
![]() NRL discovers two-dimensional waveguides February 16th, 2024
NRL discovers two-dimensional waveguides February 16th, 2024
![]() Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Three-pronged approach discerns qualities of quantum spin liquids November 17th, 2023
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








