Home > Press > Nanocrystal Catalyst Transforms Impure Hydrogen into Electricity: Brookhaven Lab scientists use simple, 'green' process to create novel core-shell catalyst that tolerates carbon monoxide in fuel cells and opens new, inexpensive pathways for zero-emission vehicles
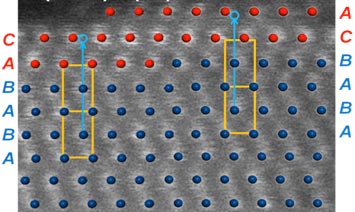 |
| Click on the image to download a high-resolution version. Computational model optimized with Density Functional Theory superimposed over a high-resolution scanning transmission electron microscopy (STEM) image (white dots). Ruthenium retains its structure with ABAB stacking sequence (blue dots) in the core, and the platinum shell switches to the distinct ABCABC stacking sequence. |
Abstract:
The quest to harness hydrogen as the clean-burning fuel of the future demands the perfect catalysts-nanoscale machines that enhance chemical reactions. Scientists must tweak atomic structures to achieve an optimum balance of reactivity, durability, and industrial-scale synthesis. In an emerging catalysis frontier, scientists also seek nanoparticles tolerant to carbon monoxide, a poisoning impurity in hydrogen derived from natural gas. This impure fuel-40 percent less expensive than the pure hydrogen produced from water-remains largely untapped.
Nanocrystal Catalyst Transforms Impure Hydrogen into Electricity: Brookhaven Lab scientists use simple, 'green' process to create novel core-shell catalyst that tolerates carbon monoxide in fuel cells and opens new, inexpensive pathways for zero-emission vehicles
Upton, NY | Posted on September 18th, 2013Now, scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory-in research published online September 18, 2013 in the journal Nature Communications-have created a high-performing nanocatalyst that meets all these demands. The novel core-shell structure-ruthenium coated with platinum-resists damage from carbon monoxide as it drives the energetic reactions central to electric vehicle fuel cells and similar technologies.
"These nanoparticles exhibit perfect atomic ordering in both the ruthenium and platinum, overcoming structural defects that previously crippled carbon monoxide-tolerant catalysts," said study coauthor and Brookhaven Lab chemist Jia Wang. "Our highly scalable, 'green' synthesis method, as revealed by atomic-scale imaging techniques, opens new and exciting possibilities for catalysis and sustainability."
Fabricating Crystals with Atomic Perfection
Catalysts inside fuel cells pry free the intrinsic energy of hydrogen molecules and convert it into electricity. Platinum performs exceptionally well with pure hydrogen fuel, but the high cost and rarity of the metal impedes its widespread deployment. By coating less expensive metals with thin layers of platinum atoms, however, scientists can retain reactivity while driving down costs and creating core-shell structures with superior performance parameters.
The carbon monoxide impurities in hydrogen formed from natural gas present another challenge to scientists because they deactivate most platinum catalysts. Ruthenium-less expensive than platinum-promotes carbon monoxide tolerance, but is more prone to dissolution during fuel cells' startup/shutdowns, causing gradual performance decay.
"We set out to protect ruthenium cores from dissolution with complete platinum shells just one or two atoms thick," Wang said. "Previous surface science studies revealed remarkable variation of surface properties in this core-shell configuration, suggesting the need and the opportunity to perfect the recipe with precise control."
Doubts existed about whether or not a highly ordered ruthenium core was even possible with a platinum shell-previously synthesized nanoparticles exhibited a weakened crystal structure in the ruthenium.
"Luckily, we found that the loss of ruthenium structure was due to defect-mediated interlayer diffusion, which is avoidable," Wang said. "By eliminating any lattice defects in ruthenium nanoparticles before adding platinum, we preserved the crucial, discrete atomic structure of each element."
The scalable and inexpensive synthesis method uses ethanol-a common and inexpensive solvent-as the reductant to fabricate the nanoparticle core and shell. The sophisticated process requires no other organic agents or metal templates.
"Simply adjusting temperature, water, and acidity of the solutions gave us complete control over the process and yielded remarkably consistent ruthenium nanoparticle size and uniform platinum coating," said Brookhaven Lab chemist Radoslav Adzic, another coauthor on the study. "This simplicity offers high reproducibility and scalability, and it demonstrates the clear commercial potential of our method."
Core-Shell Characterization
"We took the completed catalysts to other facilities here at the Lab to reveal the exact details of the atomic structure," Wang said. "This kind of rapid collaboration is only possible when you work right next door to world-class experts and instruments."
Scientists at Brookhaven Lab's National Synchrotron Light Source (NSLS) revealed the atomic density, distribution, and uniformity of the metals in the nanocatalysts using a technique called x-ray diffraction, where high-frequency light scatters and bends after interacting with individual atoms. The collaboration also used a scanning transmission electron microscope (STEM) at Brookhaven's Center for Functional Nanomaterials (CFN) to pinpoint the different sub-nanometer atomic patterns. With this instrument, a focused beam of electrons bombarded the particles, creating a map of both the core and shell structures.
"We found that the elements did not mix at the core-shell boundary, which is a critical stride," said CFN physicist Dong Su, coauthor and STEM specialist. "The atomic ordering in each element, coupled with the right theoretical models, tells us about how and why the new nanocatalyst works its magic."
Determining the ideal functional configuration for the core and shell also required the use of the CFN's expertise in computational science. With density functional theory (DFT) calculations, the computer helps identify the most energetically stable platinum-ruthenium structure.
"The DFT analysis connects the dots between performance and configuration, and it corroborates our direct observations from x-ray diffraction and electron microscopy," Adzic said.
Discovery to Deployment
Ballard Power Systems, a company dedicated to fuel cells production, independently evaluated the performance of the new core-shell nanocatalysts. Beyond testing the low-platinum catalysts' high activity in pure hydrogen, Ballard looked specifically at the resistance to carbon monoxide present in impure hydrogen gas and the dissolution resistance during startup/shutdown cycles. The bilayer nanocatalyst exhibited high durability and enhanced carbon monoxide tolerance-the combination enables the use of impure hydrogen without much loss in efficiency or increase in catalyst cost.
The nanocatalyst also performed well in producing hydrogen gas through the hydrogen evolution reaction, leading to another industrial partnership. Proton Onsite, a company specializing in splitting hydrogen from water and other similar processes, has completed feasibility tests for deploying the technology in their production of water electrolyzers, which will now require about 98 percent less platinum.
"Water electrolyzers are already on the market, so this nanocatalyst can deploy quickly," Wang said. "When hydrogen fuel cell vehicles roll out in the coming years, this new structure may accelerate development by driving down costs for both metal catalysts and fuel."
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE's Office of Science by Brookhaven Science Associates, a limited-liability company founded by the Research Foundation for the State University of New York on behalf of Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit applied science and technology organization.
The Center for Functional Nanomaterials at Brookhaven National Laboratory is one of the five DOE Nanoscale Science Research Centers (NSRCs), premier national user facilities for interdisciplinary research at the nanoscale. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos national laboratories. For more information about the DOE NSRCs, please visit http://nano.energy.gov.
The National Synchrotron Light Source (NSLS) provides intense beams of infrared, ultraviolet, and x-ray light for basic and applied research in physics, chemistry, medicine, geophysics, and environmental and materials sciences. Supported by the Office of Basic Energy Sciences within the U.S. Department of Energy, the NSLS is one of the world's most widely used scientific facilities. For more information, visit www.nsls.bnl.gov.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
For more information, please click here
Contacts:
Justin Eure
(631) 344-2347
or
Peter Genzer
(631) 344-3174
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








