Home > Press > Nanoparticle Opens the Door to Clean-Energy Alternatives
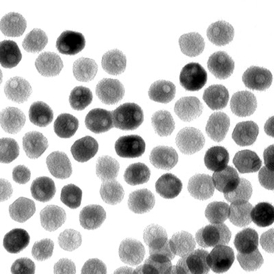 |
| A transmission-electron microscope image of a collection of quasi-spherical nickel phosphide nanoparticles. A team led by Raymond Schaak of Penn State University has found that these nanoparticles can catalyze an important chemical reaction that generates hydrogen from water. Credit: Eric Popczun, Penn State University |
Abstract:
Cheaper clean-energy technologies could be made possible thanks to a new discovery. Led by Raymond Schaak, a professor of chemistry at Penn State University, research team members have found that an important chemical reaction that generates hydrogen from water is effectively triggered -- or catalyzed -- by a nanoparticle composed of nickel and phosphorus, two inexpensive elements that are abundant on Earth. The results of the research will be published in the Journal of the American Chemical Society.
Nanoparticle Opens the Door to Clean-Energy Alternatives
University Park, PA | Posted on June 14th, 2013Schaak explained that the purpose of the nickel phosphide nanoparticle is to help produce hydrogen from water, which is a process that is important for many energy-production technologies, including fuel cells and solar cells. "Water is an ideal fuel, because it is cheap and abundant, but we need to be able to extract hydrogen from it," Schaak said. Hydrogen has a high energy density and is a great energy carrier, Schaak explained, but it requires energy to produce. To make its production practical, scientists have been hunting for a way to trigger the required chemical reactions with an inexpensive catalyst. Schaak noted that this feat is accomplished very well by platinum but, because platinum is expensive and relatively rare, he and his team have been searching for alternative materials. "There were some predictions that nickel phosphide might be a good candidate, and we had already been working with nickel phosphide nanoparticles for several years," Schaak said. "It turns out that nanoparticles of nickel phosphide are indeed active for producing hydrogen and are comparable to the best known alternatives to platinum."
To create the nickel phosphide nanoparticles, team members began with metal salts that are commercially available. They then dissolved these salts in solvents, added other chemical ingredients, and heated the solution to allow the nanoparticles to form. The researchers were able create a nanoparticle that was quasi-spherical -- not a perfect sphere, but spherical with many flat, exposed edges. "The small size of the nanoparticles creates a high surface area, and the exposed edges means that a large number of sites are available to catalyze the chemical reaction that produces hydrogen," Schaak explained.
The next step was for team members at the California Institute of Technology to test the nanoparticles' performance in catalyzing the necessary chemical reactions. Led by Nathan S. Lewis, the George L. Argyros Professor of Chemistry at the California Institute of Technology, the researchers performed these tests by placing the nanoparticles onto a sheet of titanium foil and immersing that sheet in a solution of sulfuric acid. Next, the researchers applied a voltage and measured the current produced. They found that, not only were the chemical reactions happening as they had hoped, they also were happening with a high degree of efficacy.
"Nanoparticle technology has already started to open the door to cheaper and cleaner energy that is also efficient and useful," Schaak said. "The goal now is to further improve the performance of these nanoparticles and to understand what makes them function the way they do. Also, our team members believe that our success with nickel phosphide can pave the way toward the discovery of other new catalysts that also are comprised of Earth-abundant materials. Insights from this discovery may lead to even better catalysts in the future."
In addition to Schaak and Lewis, other researchers who contributed to this study include Eric J. Popczun, Carlos G. Read, Adam J. Biacchi, and Alex M. Wiltrout from Penn State; and James R. McKone from the California Institute of Technology.
The research was funded by the U.S. National Science Foundation and the U.S. Department of Energy. The team has filed a patent application.
####
For more information, please click here
Contacts:
Raymond Schaak:
814-865-8600
Barbara Kennedy (PIO)
814-863-4682
Copyright © Penn State
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
Chemistry
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Discoveries
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Patents/IP/Tech Transfer/Licensing
![]() Getting drugs across the blood-brain barrier using nanoparticles March 3rd, 2023
Getting drugs across the blood-brain barrier using nanoparticles March 3rd, 2023
![]() Metasurfaces control polarized light at will: New research unlocks the hidden potential of metasurfaces August 13th, 2021
Metasurfaces control polarized light at will: New research unlocks the hidden potential of metasurfaces August 13th, 2021
![]() Arrowhead Pharmaceuticals Announces Closing of Agreement with Takeda November 27th, 2020
Arrowhead Pharmaceuticals Announces Closing of Agreement with Takeda November 27th, 2020
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








