Home > Press > Stanford scientists develop high-efficiency zinc-air battery
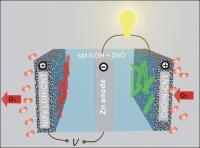 |
| This is a rechargeable zinc-oxide battery in a tri-electrode configuration with cobalt-oxide/carbon nanotube and iron-nickel/layered double hydroxide catalysts for charge and discharge, respectively.
Credit: Yanguang Li, Stanford University |
Abstract:
Stanford University scientists have developed an advanced zinc-air battery with higher catalytic activity and durability than similar batteries made with costly platinum and iridium catalysts. The results, published in the May 7 online edition of the journal Nature Communications, could lead to the development of a low-cost alternative to conventional lithium-ion batteries widely used today.
Stanford scientists develop high-efficiency zinc-air battery
Stanford, CA | Posted on May 30th, 2013"There have been increasing demands for high-performance, inexpensive and safe batteries for portable electronics, electric vehicles and other energy storage applications," said Hongjie Dai, a professor chemistry at Stanford and lead author of the study. "Metal-air batteries offer a possible low-cost solution."
According to Dai, most attention has focused on lithium-ion batteries, despite their limited energy density (energy stored per unit volume), high cost and safety problems. "With ample supply of oxygen from the atmosphere, metal-air batteries have drastically higher theoretical energy density than either traditional aqueous batteries or lithium-ion batteries," he said. "Among them, zinc-air is technically and economically the most viable option."
Zinc-air batteries combine atmospheric oxygen and zinc metal in a liquid alkaline electrolyte to generate electricity with a byproduct of zinc oxide. When the process is reversed during recharging, oxygen and zinc metal are regenerated.
"Zinc-air batteries are attractive because of the abundance and low cost of zinc metal, as well as the non-flammable nature of aqueous electrolytes, which make the batteries inherently safe to operate," Dai said. "Primary (non-rechargeable) zinc-air batteries have been commercialized for medical and telecommunication applications with limited power density. However, it remains a grand challenge to develop electrically rechargeable batteries, with the stumbling blocks being the lack of efficient and robust air catalysts, as well as the limited cycle life of the zinc electrodes."
Active and durable electrocatalysts on the air electrode are required to catalyze the oxygen-reduction reaction during discharge and the oxygen-evolution reaction during recharge. In zinc-air batteries, both catalytic reactions are sluggish, Dai said.
Recently, his group has developed a number of high-performance electrocatalysts made with non-precious metal oxide or nanocrystals hybridized with carbon nanotubes. These catalysts produced higher catalytic activity and durability in alkaline electrolytes than catalysts made with platinum and other precious metals.
"We found that similar catalysts greatly boosted the performance of zinc-air batteries," Dai said. both primary and rechargeable. "A combination of a cobalt-oxide hybrid air catalyst for oxygen reduction and a nickel-iron hydroxide hybrid air catalyst for oxygen evolution resulted in a record high-energy efficiency for a zinc-air battery, with a high specific energy density more than twice that of lithium-ion technology."
The novel battery also demonstrated good reversibility and stability over long charge and discharge cycles over several weeks. "This work could be an important step toward developing practical rechargeable zinc-air batteries, even though other challenges relating to the zinc electrode and electrolyte remain to be solved," Dai added.
###
Other authors of the Nature Communications study are Yanguang Li (lead author), Ming Gong, Yongye Liang, Ju Feng, Ji-Eun Kim, Hailiang Wang, Guosong Hong and Bo Zhang of the Stanford Department of Chemistry.
The study was supported by Intel, a Stanford Global Climate and Energy Project exploratory program and a Stinehart/Reed Award from the Stanford Precourt Institute for Energy.
This article was written by Mark Shwartz, Precourt Institute for Energy at Stanford University.
####
For more information, please click here
Contacts:
Mark Shwartz
650-723-9296
Copyright © Stanford University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Announcements
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() Next-generation quantum communication October 3rd, 2025
Next-generation quantum communication October 3rd, 2025
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Automotive/Transportation
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
Rice membrane extracts lithium from brines with greater speed, less waste October 3rd, 2025
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








