Home > Press > Movement of pyrrole molecules defy 'classical' physics
 |
Abstract:
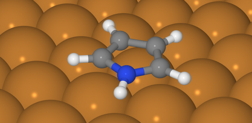
New research shows that movement of the ring-like molecule pyrrole over a metal surface runs counter to the centuries-old laws of 'classical' physics that govern our everyday world.
Movement of pyrrole molecules defy 'classical' physics
Cambridge, UK | Posted on April 28th, 2013Using uniquely sensitive experimental techniques, scientists have found that laws of quantum physics - believed primarily to influence at only sub-atomic levels - can actually impact on a molecular level.
Researchers at Cambridge's Chemistry Department and Cavendish Laboratory say they have evidence that, in the case of pyrrole, quantum laws affecting the internal motions of the molecule change the "very nature of the energy landscape" - making this 'quantum motion' essential to understanding the distribution of the whole molecule.
The study, a collaboration between scientists from Cambridge and Rutgers universities, appeared in the German chemistry journal Angewandte Chemie earlier this month.
A pyrrole molecule's centre consists of a "flat pentagram" of five atoms, four carbon and one nitrogen. Each of these atoms has an additional hydrogen atom attached, sticking out like spokes.
Following experiments performed by Barbara Lechner at the Cavendish Laboratory to determine the energy required for movement of pyrrole across a copper surface, the team discovered a discrepancy that led them down a 'quantum' road to an unusual discovery.
In previous work on simpler molecules, the scientists were able to accurately calculate the 'activation barrier' - the energy required to loosen a molecule's bond to a surface, allowing movement - using 'density functional theory', a method that treats the electrons which bind the atoms according to quantum mechanics but, crucially, deals with atomic nuclei using a 'classical' physics approach.
Surprisingly, with pyrrole the predicted 'activation barriers' were way out, with calculations "less than a third of the measured value". After much head scratching, puzzled scientists turned to a purely quantum phenomenon called 'zero-point energy'.
In classical physics, an object losing energy can continue to do so until it can be thought of as sitting perfectly still. In the quantum world, this is never the case: everything always retains some form of residual - even undetectable - energy, known as 'zero-point energy'.
While 'zero-point energy' is well known to be associated with motion of the atoms contained in molecules, it was previously believed that such tiny amounts of energy simply don't affect the molecule as a whole to any measurable extent, unless the molecule broke apart.
But now, the researchers have discovered that the "quantum nature" of the molecule's internal motion actually does affect the molecule as a whole as it moves across the surface, defying the 'classical' laws that it's simply too big to feel quantum effects.
'Zero-point energy' moving within a pyrrole molecule is unexpectedly sensitive to the exact site occupied by the molecule on the surface. In moving from one site to another, the 'activation energy' must include a sizeable contribution due to the change in the quantum 'zero-point energy'.
Scientists believe the effect is particularly noticeable in the case of pyrrole because the 'activation energy' needed for diffusion is particularly small, but that many other similar molecules ought to show the same kind of behavior.
"Understanding the nature of molecular diffusion on metal surfaces is of great current interest, due to efforts to manufacture two-dimensional networks of ring-like molecules for use in optical, electronic or spintronic devices," said Dr Stephen Jenkins, who heads up the Surface Science Group in Cambridge's Department of Chemistry.
"The balance between the activation energy and the energy barrier that sticks the molecules to the surface is critical in determining which networks are able to form under different conditions."
Credits:
Stephen Jenkins is head of the Chemistry Department's Surface Science Group; Marco Sacchi is the post-doc in that group who did the calculations.
Bill Allison and John Ellis lead the Surface Science section of the Surfaces, Microstructure and Fracture Group at the Cavendish; Holly Hedgeland was a post-doc in that group, who started a lot of the experimental work on diffusion of aromatic molecules; Barbara Lechner was the student who took the lead on the experimental work for this specific system.
Jane Hinch is a collaborator from Rutgers University, involved in the experimental work and its interpretation
####
For more information, please click here
Contacts:
Stephen Jenkins
44-012-233-36502
Copyright © University of Cambridge
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Physics
![]() Quantum computers simulate fundamental physics: shedding light on the building blocks of nature June 6th, 2025
Quantum computers simulate fundamental physics: shedding light on the building blocks of nature June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Magnetism in new exotic material opens the way for robust quantum computers June 4th, 2025
Magnetism in new exotic material opens the way for robust quantum computers June 4th, 2025
Spintronics
![]() Quantum materials: Electron spin measured for the first time June 9th, 2023
Quantum materials: Electron spin measured for the first time June 9th, 2023
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Photonics/Optics/Lasers
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Quantum nanoscience
![]() Beyond silicon: Electronics at the scale of a single molecule January 30th, 2026
Beyond silicon: Electronics at the scale of a single molecule January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








