Home > Press > Synthetic molecule first electricity-making catalyst to use iron to split hydrogen gas: Fast and efficient biologically inspired catalyst could someday make fuel cells cheaper
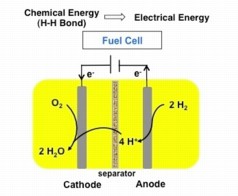 |
| Burning hydrogen in a fuel cell generates an electrical current. A new iron-based catalyst might help make those fuel cells less expensive. |
Abstract:
To make fuel cells more economical, engineers want a fast and efficient iron-based molecule that splits hydrogen gas to make electricity. Online Feb. 17 at Nature Chemistry, researchers report such a catalyst. It is the first iron-based catalyst that converts hydrogen directly to electricity. The result moves chemists and engineers one step closer to widely affordable fuel cells.
Synthetic molecule first electricity-making catalyst to use iron to split hydrogen gas: Fast and efficient biologically inspired catalyst could someday make fuel cells cheaper
Richland, WA | Posted on February 18th, 2013"A drawback with today's fuel cells is that the platinum they use is more than a thousand times more expensive than iron," said chemist R. Morris Bullock, who leads the research at the Department of Energy's Pacific Northwest National Laboratory.
His team at the Center for Molecular Electrocatalysis has been developing catalysts that use cheaper metals such as nickel and iron. The one they report here can split hydrogen as fast as two molecules per second with an efficiency approaching those of commercial catalysts. The center is one of 46 Energy Frontier Research Centers established by the DOE Office of Science across the nation in 2009 to accelerate basic research in energy.
Fuel cells generate electricity out of a chemical fuel, usually hydrogen. The bond within a hydrogen molecule stores electricity, where two electrons connect two hydrogen atoms like a barbell.
Fuel cells use a platinum catalyst -- essentially a chunk of metal -- to crack a hydrogen molecule open like an egg: The electron whites run out and form a current that is electricity. Because platinum's chemical nature gives it the ability to do this, chemists can't simply replace the expensive metal with the cheaper iron or nickel. However, a molecule that exists in nature called a hydrogenase (high-dra-jin-ace) uses iron to split hydrogen.
Bullock and his PNNL colleagues, chemists Tianbiao "Leo" Liu and Dan DuBois, have taken inspiration for their iron-wielding catalyst from a hydrogenase. First Liu created several potential molecules for the team to test. Then, with the best-working molecule up to that point, they determined and tweaked the shape and the internal electronic forces to make additional improvements.
One of the tricks they needed the catalyst to do was to split hydrogen atoms into all of their parts. If a hydrogen atom is an egg, the positively charged proton that serves as the nucleus of the atom would be the yolk. And the electron, which orbits around the proton in a cloud, would be the white. The catalyst moves both the proton-yolks and electron-whites around in a controlled series of steps, sending the protons in one direction and the electrons to an electrode, where the electricity can be used to power things.
To do this, they need to split hydrogen molecules unevenly in an early step of the process. One hydrogen molecule is made up of two protons and two electrons, but the team needed the catalyst to tug away one proton first and send it away, where it is caught by a kind of molecule called a proton acceptor. In a real fuel cell, the acceptor would be oxygen.
Once the first proton with its electron-wooing force is gone, the electrode easily plucks off the first electron. Then another proton and electron are similarly removed, with both of the electrons being shuttled off to the electrode.
The team determined the shape and size of the catalyst and also tested different proton acceptors. With the iron in the middle, arms hanging like pendants around the edges draw out the protons. The best acceptors stole these drawn-off protons away quickly.
With their design down, the team measured how fast the catalyst split molecular hydrogen. It peaked at about two molecules per second, thousands of times faster than the closest, non-electricity making iron-based competitor. In addition, they determined its overpotential, which is a measure of how efficient the catalyst is. Coming in at 160 to 220 millivolts, the catalyst revealed itself to be similar in efficiency to most commercially available catalysts.
Now the team is figuring out the slow steps so they can make them faster, as well as determining the best conditions under which this catalyst performs.
This work was supported by the Department of Energy, Office of Science.
Reference: Tianbiao Liu, Daniel L. DuBois and R. Morris Bullock. An iron complex with pendent amines as a molecular electrocatalyst for oxidation of hydrogen, Nature Chemistry, Month Day, 2013, doi:10.1038/NCHEM.1571.
####
About DOE/Pacific Northwest National Laboratory
Interdisciplinary teams at Pacific Northwest National Laboratory address many of America's most pressing issues in energy, the environment and national security through advances in basic and applied science. PNNL employs 4,500 staff, has an annual budget of nearly $1 billion, and has been managed for the U.S. Department of Energy by Ohio-based Battelle since the laboratory's inception in 1965. For more, visit the PNNL's News Center, or follow PNNL on Facebook, LinkedIn and Twitter.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit the Office of Science website at science.energy.gov.
For more information, please click here
Contacts:
Mary Beckman
509-375-3688
Copyright © DOE/Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








