Home > Press > Ions, not particles, make silver toxic to bacteria: Rice University researchers report too small a dose may enhance microbes’ immunity
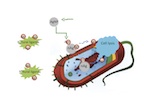 |
| Silver ions delivered by nanoparticles to bacteria promote lysis, the process by which cells break down and ultimately die, which makes silver nanoparticles a superior and widely used antibacterial agent. New research by Rice University found that silver ions, not the particles themselves, are toxic to bacteria. They also found that ligands in the vicinity of a bacteria can bind silver ions and prevent them from reaching their target. (Credit: Zongming Xiu/Rice University) |
Abstract:
Rice University researchers have settled a long-standing controversy over the mechanism by which silver nanoparticles, the most widely used nanomaterial in the world, kill bacteria.
Ions, not particles, make silver toxic to bacteria: Rice University researchers report too small a dose may enhance microbes’ immunity
Houston, TX | Posted on July 11th, 2012Their work comes with a Nietzsche-esque warning: Use enough. If you don't kill them, you make them stronger.
Scientists have long known that silver ions, which flow from nanoparticles when oxidized, are deadly to bacteria. Silver nanoparticles are used just about everywhere, including in cosmetics, socks, food containers, detergents, sprays and a wide range of other products to stop the spread of germs.
But scientists have also suspected silver nanoparticles themselves may be toxic to bacteria, particularly the smallest of them at about 3 nanometers. Not so, according to the Rice team that reported its results this month in the American Chemical Society journal Nano Letters.
In fact, when the possibility of ionization is taken away from silver, the nanoparticles are practically benign in the presence of microbes, said Pedro Alvarez, George R. Brown Professor and chair of Rice's Civil and Environmental Engineering Department.
"You would be surprised how often people market things without a full mechanistic understanding of their function," said Alvarez, who studies the fate of nanoparticles in the environment and their potential toxicity, particularly to humans. "The prefix ‘nano' can be a double-edged sword. It can help you sell a product, and in other cases it might elicit concerns about potential unintended consequences."
He said the straightforward answer to the decade-old question is that the insoluble silver nanoparticles do not kill cells by direct contact. But soluble ions, when activated via oxidation in the vicinity of bacteria, do the job nicely.
To figure that out, the researchers had to strip the particles of their powers. "Our original expectation was that the smaller a particle is, the greater the toxicity," said Zongming Xiu, a Rice postdoctoral researcher and lead author of the paper. Xiu set out to test nanoparticles, both commercially available and custom-synthesized from 3 to 11 nanometers, to see whether there was a correlation between size and toxicity.
"We could not get consistent results," he said. "It was very frustrating and really weird."
Xiu decided to test nanoparticle toxicity in an anaerobic environment - that is, sealed inside a chamber with no exposure to oxygen — to control the silver ions' release. He found that the filtered particles were a lot less toxic to microbes than silver ions.
Working with the lab of Rice chemist Vicki Colvin, the team then synthesized silver nanoparticles inside the anaerobic chamber to eliminate any chance of oxidation. "We found the particles, even up to a concentration of 195 parts per million, were still not toxic to bacteria," Xiu said. "But for the ionic silver, a concentration of about 15 parts per billion would kill all the bacteria present. That told us the particle is 7,665 times less toxic than the silver ions, indicating a negligible toxicity."
"The point of that experiment," Alvarez said, "was to show that a lot of people were obtaining data that was confounded by a release of ions, which was occurring during exposure they perhaps weren't aware of."
Alvarez suggested the team's anaerobic method may be used to test many other kinds of metallic nanoparticles for toxicity and could help fine-tune the antibacterial qualities of silver particles. In their tests, the Rice researchers also found evidence of hormesis; E. coli became stimulated by silver ions when they encountered doses too small to kill them.
"Ultimately, we want to control the rate of (ion) release to obtain the desired concentrations that just do the job," Alvarez said. "You don't want to overshoot and overload the environment with toxic ions while depleting silver, which is a noble metal, a valuable resource - and a somewhat expensive disinfectant. But you don't want to undershoot, either."
He said the finding should shift the debate over the size, shape and coating of silver nanoparticles. "Of course they matter," Alvarez said, "but only indirectly, as far as these variables affect the dissolution rate of the ions. The key determinant of toxicity is the silver ions. So the focus should be on mass-transfer processes and controlled-release mechanisms."
"These findings suggest that the antibacterial application of silver nanoparticles could be enhanced and environmental impacts could be mitigated by modulating the ion release rate, for example, through responsive polymer coatings," Xiu said.
Co-authors of the paper are postdoctoral researcher Qingbo Zhang and graduate student Hema Puppala, both in the lab of Colvin, Rice's Kenneth S. Pitzer-Schlumberger Professor of Chemistry, a professor of chemical and biomolecular engineering and vice provost for research.
The work was supported by a joint U.S.-U.K. research program administered by the Environmental Protection Agency and the U.K.'s Natural Environment Research Council.
####
About Rice University
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is known for its “unconventional wisdom.” With 3,708 undergraduates and 2,374 graduate students, Rice’s undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 4 for “best value” among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go to www.rice.edu/nationalmedia/Rice.pdf.
For more information, please click here
Contacts:
Jeff Falk
713-348-6775
Mike Williams
713-348-6728
Copyright © Rice University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Safety-Nanoparticles/Risk management
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
![]() Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Billions of nanoplastics released when microwaving baby food containers: Exposure to plastic particles kills up to 75% of cultured kidney cells July 21st, 2023
Nanobiotechnology
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








