Home > Press > Nanotube 'sponge' has potential in oil spill cleanup
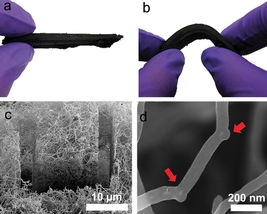 |
| A carbon nanotube sponge developed with help from ORNL researchers holds potential as an aid for oil spill cleanup. Simulations at ORNL explained how the addition of boron atoms encouraged the formation of so-called "elbow" junctions that help the nanotubes grow into a 3-D network. |
Abstract:
A carbon nanotube sponge that can soak up oil in water with unparalleled efficiency has been developed with help from computational simulations performed at the Department of Energy's (DOE's) Oak Ridge National Laboratory.
Nanotube 'sponge' has potential in oil spill cleanup
Oak Ridge, TN | Posted on May 10th, 2012Carbon nanotubes, which consist of atom-thick sheets of carbon rolled into cylinders, have captured scientific attention in recent decades because of their high strength, potential high conductivity and light weight. But producing nanotubes in bulk for specialized applications was often limited by difficulties in controlling the growth process as well as dispersing and sorting the produced nanotubes.
ORNL's Bobby Sumpter was part of a multi-institutional research team that set out to grow large clumps of nanotubes by selectively substituting boron atoms into the otherwise pure carbon lattice. Sumpter and Vincent Meunier, now of Rensselaer Polytechnic Institute, conducted simulations on supercomputers, including Jaguar at ORNL's Leadership Computing Facility, to understand how the addition of boron would affect the carbon nanotube structure.
"Any time you put a different atom inside the hexagonal carbon lattice, which is a chicken wire-like network, you disrupt that network because those atoms don't necessarily want to be part of the chicken wire structure," Sumpter said. "Boron has a different number of valence electrons, which results in curvature changes that trigger a different type of growth."
Simulations and lab experiments showed that the addition of boron atoms encouraged the formation of so-called "elbow" junctions that help the nanotubes grow into a 3-D network. The team's results are published in Nature Scientific Reports.
"Instead of a forest of straight tubes, you create an interconnected, woven sponge-like material," Sumpter said. "Because it is interconnected, it becomes three-dimensionally strong, instead of only one-dimensionally strong along the tube axis."
Further experiments showed the team's material, which is visible to the human eye, is extremely efficient at absorbing oil in contaminated seawater because it attracts oil and repels water.
"It loves carbon because it is primarily carbon," Sumpter said. "Depending on the density of oil to water content and the density of the sponge network, it will absorb up to 100 times its weight in oil."
The material's mechanical flexibility, magnetic properties, and strength lend it additional appeal as a potential technology to aid in oil spill cleanup, Sumpter says.
"You can reuse the material over and over again because it's so robust," he said. "Burning it does not substantially decrease its ability to absorb oil, and squeezing it like a sponge doesn't damage it either."
The material's magnetic properties, caused by the team's use of an iron catalyst during the nanotube growth process, means it can be easily controlled or removed with a magnet in an oil cleanup scenario. This ability is an improvement over existing substances used in oil removal, which are often left behind after cleanup and can degrade the environment.
The experimental team has submitted a patent application on the technology through Rice University. The research is published as "Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions," and is available online here: www.nature.com/srep/2012/120413/srep00363/full/srep00363.html.
The research team included researchers from ORNL, Rice University; Universidade de Vigo, Spain; Rensselaer Polytechnic Institute; University of Illinois at Urbana-Champaign; Instituto de Microelectronica de Madrid, Spain; Air Force Office of Scientific Research Laboratory; Arizona State University; Universite Catholique de Louvain, Belgium; The Pennsylvania State University; and Shinshu University, Japan.
The work was supported by the National Science Foundation, the U.S. Air Force Office of Scientific Research, the U.S. Army Research Laboratory, and by the DOE Office of Science through ORNL's Center for Nanophase Materials Sciences (CNMS) and the laboratory's Leadership Computing Facility.
CNMS is one of the five DOE Nanoscale Science Research Centers supported by the DOE Office of Science, premier national user facilities for interdisciplinary research at the nanoscale. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos national laboratories. For more information about the DOE NSRCs, please visit science.energy.gov/bes/suf/user-facilities/nanoscale-science-research-centers/.
####
About Oak Ridge National Laboratory
ORNL is managed by UT-Battelle for the Department of Energy's Office of Science. DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
For more information, please click here
Contacts:
Morgan McCorkle
Communications and Media Relations
865.574.7308
Copyright © Oak Ridge National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings/Nanosheets
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Environment
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








