Home > Press > Say Hello to Cheaper Hydrogen Fuel Cells: Los Alamos scientists document utility of non-precious-metal catalysts
 |
Abstract:
Los Alamos National Laboratory scientists have developed a way to avoid the use of expensive platinum in hydrogen fuel cells, the environmentally friendly devices that might replace current power sources in everything from personal data devices to automobiles.
Say Hello to Cheaper Hydrogen Fuel Cells: Los Alamos scientists document utility of non-precious-metal catalysts
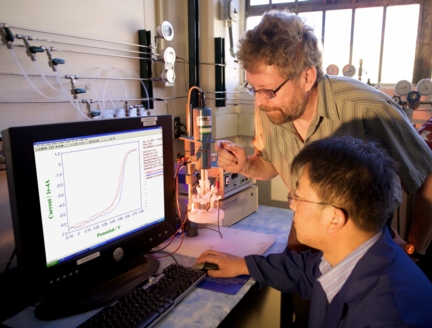
Los Alamos, NM | Posted on April 22nd, 2011In a paper published today in Science, Los Alamos researchers Gang Wu, Christina Johnston, and Piotr Zelenay, joined by researcher Karren More of Oak Ridge National Laboratory, describe the use of a platinum-free catalyst in the cathode of a hydrogen fuel cell. Eliminating platinum—a precious metal more expensive than gold—would solve a significant economic challenge that has thwarted widespread use of large-scale hydrogen fuel cell systems.
Polymer-electrolyte hydrogen fuel cells convert hydrogen and oxygen into electricity. The cells can be enlarged and combined in series for high-power applications, including automobiles. Under optimal conditions, the hydrogen fuel cell produces water as a "waste" product and does not emit greenhouse gasses. However, because the use of platinum in catalysts is necessary to facilitate the reactions that produce electricity within a fuel cell, widespread use of fuel cells in common applications has been cost prohibitive. An increase in the demand for platinum-based catalysts could drive up the cost of platinum even higher than its current value of nearly $1,800 an ounce.
The Los Alamos researchers developed non-precious-metal catalysts for the part of the fuel cell that reacts with oxygen. The catalysts—which use carbon (partially derived from polyaniline in a high-temperature process), and inexpensive iron and cobalt instead of platinum—yielded high power output, good efficiency, and promising longevity. The researchers found that fuel cells containing the carbon-iron-cobalt catalyst synthesized by Wu not only generated currents comparable to the output of precious-metal-catalyst fuel cells, but held up favorably when cycled on and off—a condition that can damage inferior catalysts relatively quickly.
Moreover, the carbon-iron-cobalt catalyst fuel cells effectively completed the conversion of hydrogen and oxygen into water, rather than producing large amounts of undesirable hydrogen peroxide. Inefficient conversion of the fuels, which generates hydrogen peroxide, can reduce power output by up to 50 percent, and also has the potential to destroy fuel cell membranes. Fortunately, the carbon- iron-cobalt catalysts synthesized at Los Alamos create extremely small amounts of hydrogen peroxide, even when compared with state-of-the-art platinum-based oxygen-reduction catalysts.
Because of the successful performance of the new catalyst, the Los Alamos researchers have filed a patent for it.
"The encouraging point is that we have found a catalyst with a good durability and life cycle relative to platinum-based catalysts," said Zelenay, corresponding author for the paper. "For all intents and purposes, this is a zero-cost catalyst in comparison to platinum, so it directly addresses one of the main barriers to hydrogen fuel cells."
The next step in the team's research will be to better understand the mechanism underlying the carbon-iron-cobalt catalyst. Micrographic images of portions of the catalyst by researcher More have provided some insight into how it functions, but further work must be done to confirm theories by the research team. Such an understanding could lead to improvements in non-precious-metal catalysts, further increasing their efficiency and lifespan.
Project funding for the Los Alamos research came from the U.S. Department of Energy's Energy Efficiency and Renewable Energy (EERE) Office as well as from Los Alamos National Laboratory's Laboratory-Directed Research and Development program. Microscopy research was done at Oak Ridge National Laboratory's SHaRE user facility with support from the DOE's Office of Basic Energy Sciences.
####
About Los Alamos National Laboratory
Los Alamos National Laboratory (LANL), a multidisciplinary research institution engaged in strategic science on behalf of national security, is operated by Los Alamos National Security, LLC, a team composed of Bechtel National, the University of California, The Babcock & Wilcox Company, and URS for the Department of Energy’s National Nuclear Security Administration.
LANL enhances national security by ensuring the safety and reliability of the U.S. nuclear stockpile, developing technologies to reduce threats from weapons of mass destruction, and solving problems related to energy, environment, infrastructure, health, and global security concerns.
For more information, please click here
Contacts:
JAMES E. RICKMAN
505-665-9203
Copyright © Los Alamos National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








