Home > Press > Berkeley Lab Scientists Achieve Breakthrough in Nanocomposite for High-Capacity Hydrogen Storage
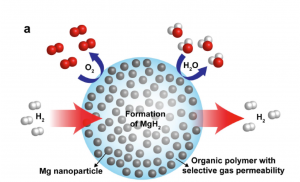 |
| This schematic shows high-capacity magnesium nanocrystals encapsulated in a gas-barrier polymer matrix to create a new and revolutionary hydrogen storage composite material. (Image from Jeff Urban) |
Abstract:
Since the 1970s, hydrogen has been touted as a promising alternative to fossil fuels due to its clean combustion —unlike the combustion of fossil fuels, which spews greenhouse gases and harmful pollutants, hydrogen's only combustion by-product is water. Compared to gasoline, hydrogen is lightweight, can provide a higher energy density and is readily available. But there's a reason we're not already living in a hydrogen economy: to replace gasoline as a fuel, hydrogen must be safely and densely stored, yet easily accessed. Limited by materials unable to leap these conflicting hurdles, hydrogen storage technology has lagged behind other clean energy candidates.
Berkeley Lab Scientists Achieve Breakthrough in Nanocomposite for High-Capacity Hydrogen Storage
Berkeley, CA | Posted on March 14th, 2011In recent years, researchers have attempted to tackle both issues by locking hydrogen into solids, packing larger quantities into smaller volumes with low reactivity—a necessity in keeping this volatile gas stable. However, most of these solids can only absorb a small amount of hydrogen and require extreme heating or cooling to boost their overall energy efficiency.
Now, scientists with the U.S. Department of Energy (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) have designed a new composite material for hydrogen storage consisting of nanoparticles of magnesium metal sprinkled through a matrix of polymethyl methacrylate, a polymer related to Plexiglas. This pliable nanocomposite rapidly absorbs and releases hydrogen at modest temperatures without oxidizing the metal after cycling—a major breakthrough in materials design for hydrogen storage, batteries and fuel cells.
"This work showcases our ability to design composite nanoscale materials that overcome fundamental thermodynamic and kinetic barriers to realize a materials combination that has been very elusive historically," says Jeff Urban, Deputy Director of the Inorganic Nanostructures Facility at the Molecular Foundry, a DOE Office of Science nanoscience center and national user facility located at Berkeley Lab. "Moreover, we are able to productively leverage the unique properties of both the polymer and nanoparticle in this new composite material, which may have broad applicability to related problems in other areas of energy research."
Urban, along with coauthors Ki-Joon Jeon and Christian Kisielowski used the TEAM 0.5 microscope at the National Center for Electron Microscopy (NCEM), another DOE Office of Science national user facility housed at Berkeley Lab, to observe individual magnesium nanocrystals dispersed throughout the polymer. With the high-resolution imaging capabilities of TEAM 0.5, the world's most powerful electron microscope, the researchers were also able to track defects—atomic vacancies in an otherwise-ordered crystalline framework—providing unprecedented insight into the behavior of hydrogen within this new class of storage materials.
"Discovering new materials that could help us find a more sustainable energy solution is at the core of the Department of Energy's mission. Our lab provides outstanding experiments to support this mission with great success," says Kisielowski. "We confirmed the presence of hydrogen in this material through time-dependent spectroscopic investigations with the TEAM 0.5 microscope. This investigation suggests that even direct imaging of hydrogen columns in such materials can be attempted using the TEAM microscope."
"The unique nature of Berkeley Lab encourages cross-division collaborations without any limitations," said Jeon, now at the Ulsan National Institute of Science and Technology, whose postdoctoral work with Urban led to this publication
To investigate the uptake and release of hydrogen in their nanocomposite material, the team turned to Berkeley Lab's Energy and Environmental Technologies Division (EETD), whose research is aimed at developing more environmentally friendly technologies for generating and storing energy, including hydrogen storage.
"Here at EETD, we have been working closely with industry to maintain a hydrogen storage facility as well as develop hydrogen storage property testing protocols," says Samuel Mao, director of the Clean Energy Laboratory at Berkeley Lab and an adjunct engineering faculty member at the University of California (UC), Berkeley. "We very much enjoy this collaboration with Jeff and his team in the Materials Sciences Division, where they developed and synthesized this new material, and were then able to use our facility for their hydrogen storage research."
Adds Urban, "This ambitious science is uniquely well-positioned to be pursued within the strong collaborative ethos here at Berkeley Lab. The successes we achieve depend critically upon close ties between cutting-edge microscopy at NCEM, tools and expertise from EETD, and the characterization and materials know-how from MSD."
This research is reported in a paper titled, "Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without heavy metal catalysts," appearing in the journal Nature Materials and available in Nature Materials online. Co-authoring the paper with Urban, Kisielowski and Jeon were Hoi Ri Moon, Anne M. Ruminski, Bin Jiang and Rizia Bardhan.
This work was supported by DOE's Office of Science.
The Molecular Foundry is one of the five DOE Nanoscale Science Research Centers (NSRCs), premier national user facilities for interdisciplinary research at the nanoscale. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos National Laboratories.
####
About Berkeley Lab
Lawrence Berkeley National Laboratory is a U.S. Department of Energy (DOE) national laboratory managed by the University of California for the DOE Office of Science. Berkeley Lab provides solutions to the world’s most urgent scientific challenges including sustainable energy, climate change, human health, and a better understanding of matter and force in the universe. It is a world leader in improving our lives through team science, advanced computing, and innovative technology.
For more information, please click here
Contacts:
Aditi Risbud
(510)486-4861
Copyright © Berkeley Lab
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() For more information about the Molecular Foundry vist the Website at
For more information about the Molecular Foundry vist the Website at
![]() For more information about the National Center for Electron Microscopy Center visit the Website at
For more information about the National Center for Electron Microscopy Center visit the Website at
![]() For more information about the DOE Hydrogen Storage program, please visit:
For more information about the DOE Hydrogen Storage program, please visit:
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discoveries
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Utilizing palladium for addressing contact issues of buried oxide thin film transistors April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








