Home > Press > Novel Cancer-Targeting Investigational Nanoparticle Receives FDA IND Approval for First-in-Human Trial
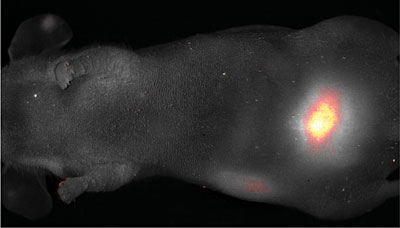 |
| C dots fluoresce brightly enough to be seen through the skin of a mouse (faintly visible in this photo). Dots coated with polyethylene glycol have all reached the bladder in 45 minutes, demonstrating that C dots will be harmlessly excreted after they do their job. Credit Memorial Sloan Kettering Cancer Center |
Abstract:
Researchers at Memorial Sloan-Kettering Cancer Center's Nanotechnology Center, along with collaborators at Cornell University and Hybrid Silica Technologies, have received approval for their first Investigational New Drug Application (IND) from the US Food and Drug Administration (FDA) for an ultrasmall silica inorganic nanoparticle platform for targeted molecular imaging of cancer, which may be useful for cancer treatment in the future.
Novel Cancer-Targeting Investigational Nanoparticle Receives FDA IND Approval for First-in-Human Trial
New York, NY | Posted on January 31st, 2011Center researchers are about to launch their first-in-human clinical trial in melanoma patients using this first-of-its-kind inorganic nanoparticle to be approved as a drug. "This is a very exciting and important first step for this new particle technology that we hope will ultimately lead to significant improvements in patient outcomes and prognoses for a number of different cancers," said Michelle Bradbury, MD, PhD, a clinician-scientist on Memorial Sloan-Kettering's Neuroradiology Service and an assistant professor of radiology at Weill Cornell Medical College, who is the lead investigator of the study, along with Snehal Patel, MD, a surgeon on Memorial Sloan-Kettering's Head and Neck Service, who is a co-principal investigator.
Cornell dots, or C dots, were initially developed as optical probes at Cornell University, Ithaca, by Ulrich Wiesner, PhD, a professor of materials science and engineering who, along with Hybrid Silica Technologies, Inc., the supplier of C dots, has spent the past eight years precisely engineering these particles. C dots were subsequently modified at Memorial Sloan-Kettering for use in PET imaging. C dots are tiny silica spheres that contain dye that glows three times more brightly than simple free dyes when excited by light of a specific wavelength. C dots can "light up" cancer cells, and act as tumor tracers for tracking the movement of cells and assisting in the optical diagnosis of tumors near the skin surface. The attachment of a radioactive label produces a new generation of multimodal (PET-optical) particle probes that additionally enable deeper detection, imaging, and monitoring of drug delivery using three-dimensional PET techniques.
C dots can be tailored to any particle size. Previous imaging experiments in mice conducted by the Memorial Sloan-Kettering team showed that particles of a very small size (in the 5 to 7 nanometer range) could be retained in the bloodstream and efficiently cleared through the kidneys after applying a neutral surface coat. More recently, the research team molecularly customized C dots to create a new particle platform, or probe, that can target surface receptors or other molecules expressed on tumor surfaces and that can be cleared through the kidneys. Using PET scans, C dots can be imaged to evaluate various biological properties of the tumors, including tumor accumulation, spread of metastatic disease, and treatment response to therapy.
The information gained from imaging tumors targeted with this multimodal platform may ultimately assist physicians in determining the extent of a tumor's spread, mapping lymph node disease, defining tumor borders for surgery, and improving real-time visualization of small vascular beds, anatomic channels, and neural structures during surgery.
The purpose of this trial is to evaluate the distribution, tissue, uptake, and safety of the particles in humans by PET imaging. This study will provide data that will serve as a baseline to guide the design of future surgical and oncologic applications in the clinic. "The use of PET imaging is an ideal imaging technology for sensitively monitoring very small doses of this new particle probe in first-in-human trials," added Steven Larson, MD, Chief of Memorial Sloan-Kettering's Nuclear Medicine Service.
Memorial Sloan-Kettering nanochemist Oula Penate Medina, PhD, notes that "this is an important trial in that it will help to answer a number of key questions regarding future potential applications of this multimodal system. Once the door has been opened, new and emerging fields, such as targeted drug delivery, can be investigated. We expect that these particles can be adapted for multiple clinical uses, including the early diagnosis and treatment of various cancers, as well as for sensing changes in the microenvironment."
"This clinical trial is the culmination of a longstanding collaborative effort with our colleagues at Cornell and Hybrid Silica Technologies, as well as a testament to our own institutional colleagues here at the Center," Dr. Bradbury said. "With the support of many, in particular the Office of Clinical Research, we've pushed to translate the C dots from a laboratory idea to our first FDA IND-approved inorganic nanomedicine drug product to be tested in the clinic," Dr. Bradbury said.
The work was funded in part by the Clinical and Translational Science Center, Weill Cornell Medical College, the Cornell Nanobiology Center, and the NIH Small-Animal Imaging Research Program (SAIRP). In addition to Drs. Bradbury, Penante-Medina, Larson, Patel, and Wiesner, the following Memorial Sloan-Kettering investigators contributed to and/or supported this work: Pat Zanzonico, PhD; Heiko Schöder, MD; Elisa De Stanchina, PhD; Hedvig Hricak, MD, Chair of the Department of Radiology; as well as Hooisweng Ow of Hybrid Silica Technologies, Inc.; Memorial Sloan-Kettering's Office of Clinical Research; and the Cyclotron Core.
####
About Memorial Sloan-Kettering Cancer Center
Memorial Sloan-Kettering Cancer Center is the world’s oldest and largest private institution devoted to prevention, patient care, research, and education in cancer. Our scientists and clinicians generate innovative approaches to better understand, diagnose, and treat cancer. These specialists are leaders in biomedical research and in translating the latest research to advance the standard of cancer care worldwide.
For more information, please click here
Contacts:
Jeanne D'Agostino
212-639-3573
Copyright © Memorial Sloan-Kettering Cancer Center
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Nanomedicine
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Quantum Dots/Rods
![]() A new kind of magnetism November 17th, 2023
A new kind of magnetism November 17th, 2023
![]() IOP Publishing celebrates World Quantum Day with the announcement of a special quantum collection and the winners of two prestigious quantum awards April 14th, 2023
IOP Publishing celebrates World Quantum Day with the announcement of a special quantum collection and the winners of two prestigious quantum awards April 14th, 2023
![]() Qubits on strong stimulants: Researchers find ways to improve the storage time of quantum information in a spin rich material January 27th, 2023
Qubits on strong stimulants: Researchers find ways to improve the storage time of quantum information in a spin rich material January 27th, 2023
![]() NIST’s grid of quantum islands could reveal secrets for powerful technologies November 18th, 2022
NIST’s grid of quantum islands could reveal secrets for powerful technologies November 18th, 2022
Nanobiotechnology
![]() New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
New micromaterial releases nanoparticles that selectively destroy cancer cells April 5th, 2024
![]() Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
Good as gold - improving infectious disease testing with gold nanoparticles April 5th, 2024
![]() Researchers develop artificial building blocks of life March 8th, 2024
Researchers develop artificial building blocks of life March 8th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








