Home > Press > New Highly Stable Fuel-Cell Catalyst Gets Strength from its Nano Core
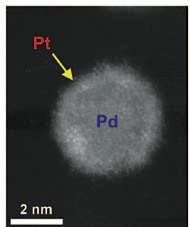 |
| This high-angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) image shows a bright shell on a relatively darker nanoparticle, signifying the formation of a core/shell structure — a platinum monolayer shell on a palladium nanoparticle core. |
Abstract:
Palladium core protects precious platinum; enhances reactivity/stability
New Highly Stable Fuel-Cell Catalyst Gets Strength from its Nano Core
Upton, NY | Posted on November 10th, 2010Stop-and-go driving can wear on your nerves, but it really does a number on the precious platinum that drives reactions in automotive fuel cells. Before large fleets of fuel-cell-powered vehicles can hit the road, scientists will have to find a way to protect the platinum, the most expensive component of fuel-cell technology, and to reduce the amount needed to make catalytically active electrodes.
Now, scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory have developed a new electrocatalyst that uses a single layer of platinum and minimizes its wear and tear while maintaining high levels of reactivity during tests that mimic stop-and-go driving. The research — described online in Angewandte Chemie, International Edition, and identified by the journal as a "very important paper" — may greatly enhance the practicality of fuel-cell vehicles and may also be applicable for improving the performance of other metallic catalysts.
The newly designed catalysts are composed of a single layer of platinum over a palladium (or palladium-gold alloy) nanoparticle core. Their structural characterization was performed at Brookhaven's Center for Functional Nanomaterials and the National Synchrotron Light Source.
"Our studies of the structure and activity of this catalyst — and comparisons with platinum-carbon catalysts currently in use — illustrate that the palladium core ‘protects' the fine layer of platinum surrounding the particles, enabling it to maintain reactivity for a much longer period of time," explained Brookhaven Lab chemist Radoslav Adzic, who leads the research team.
In conventional fuel-cell catalysts, the oxidation and reduction cycling — triggered by changes in voltage that occur during stop-and-go driving — damages the platinum. Over time, the platinum dissolves, causing irreversible damage to the fuel cell.
In the new catalyst, palladium from the core is more reactive than platinum in these oxidation and reduction reactions. Stability tests simulating fuel cell voltage cycling revealed that, after 100,000 potential cycles, a significant amount of palladium had been oxidized, dissolved, and migrated away from the cathode. In the membrane between the cathode and anode, the dissolved palladium ions were reduced by hydrogen diffusing from the anode to form a "band," or dots.
In contrast, platinum was almost unaffected, except for a small contraction of the platinum monolayer. "This contraction of the platinum lattice makes the catalyst more active and the stability of the particles increases," Adzic said.
Reactivity of the platinum monolayer/palladium core catalyst also remained extremely high. It was reduced by merely 37 percent after 100,000 cycles.
Building on earlier work that illustrated how small amounts of gold can enhance catalytic activity, the scientists also developed a form of the platinum monolayer catalyst with a palladium-gold alloy core. The addition of gold further increased the stability of the electrocatalyst, which retained nearly 70 percent of reactivity after 200,000 cycles of testing.
"This indicates the excellent durability of this electrocatalyst, especially when compared with simpler platinum-carbon catalysts, which lose nearly 70 percent of their reactivity after much shorter cycling times. This level of activity and stability indicates that this is a practical catalyst. It exceeds the goal set by DOE for 2010-2015 and it can be used for automotive applications," Adzic said.
He noted that fuel cells made using the new catalyst would require only about 10 grams of platinum per car — and less than 20 grams of palladium. Currently, in catalytic convertors used to treat exhaust gases, 5 to 10 grams of platinum is used. Since fuel-cell-powered cars would emit no exhaust gases, there would be no need for such catalytic converters, and therefore no net increase in the amount of platinum used.
"In addition to developing electrocatalysts for automotive fuel cell applications, these findings indicate the broad applicability of platinum monolayer catalysts and the possibility of extending this concept to catalysts based on other noble metals," Adzic said.
The fundamental science leading to the development of the new electrocatalyst and early scale-up work was funded by the DOE Office of Science. Additional funding came from the Toyota Motor Corporation.
The Center for Functional Nanomaterials at Brookhaven National Laboratory is one of the five DOE Nanoscale Science Research Centers (NSRCs), premier national user facilities for interdisciplinary research at the nanoscale. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE's Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge and Sandia and Los Alamos national laboratories.
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE's Office of Science by Brookhaven Science Associates, a limited-liability company founded by the Research Foundation of State University of New York on behalf of Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization.
For more information, please click here
Contacts:
Karen McNulty Walsh
(631) 344-8350
Peter Genzer
(631) 344-3174
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Chemistry
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Nanoscale CL thermometry with lanthanide-doped heavy-metal oxide in TEM March 8th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Chemical reactions can scramble quantum information as well as black holes April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








