Home > Press > Saving Platinum
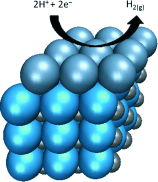 |
| How low can you go? The lower limits of platinum loading, in the sub-monolayer to monolayer range, are explored for the hydrogen evolution reaction (HER). A low-cost substrate material, tungsten monocarbide (see picture; W blue, C small gray spheres) is capable of supporting monolayer amounts of platinum (large blue-gray spheres) to produce an electrocatalyst with the same HER activity as bulk platinum. |
Abstract:
Monolayer of platinum atoms on a tungsten carbide support catalyzes the electrolytic production of hydrogen effectively and cheaply
Saving Platinum
Weinheim, Germany | Posted on October 15th, 2010Hydrogen is one of the most promising fuels of the future. Whether powered by wind or sun energy, electrolysis of water is the method of choice for producing hydrogen without emission of carbon dioxide. The character and properties of the hydrogen-producing catalyst, usually platinum, are of critical importance for the efficiency and cost of the electrocatalytic system. In the journal Angewandte Chemie, Jingguang G. Chen and a team at the University of Delaware (USA) have now introduced a new method for saving on platinum without losing efficiency: they deposit a single layer of platinum atoms onto an inexpensive tungsten carbide support.
Splitting water by electrolysis to produce hydrogen only works efficiently if the cathode, the cell's negative electrode, is equipped with an efficient catalyst. Platinum is the material of choice because of its high activity—unfortunately it is very expensive, currently costing around 52 dollars a gram. "Its high price and limited availability are the biggest stumbling blocks on the way to the mass production of hydrogen through electrolysis," explains Chen.
Current attempts to save on platinum by depositing platinum particles onto a support, have not been efficient enough. The platinum atoms often settle too far inside the porous support and are shielded from the reaction. Says Chen, "Our aim was to deposit a single layer of platinum atoms onto an inexpensive planar support so that all the platinum atoms can participate in the reaction."
The problem with this method is that if such a monolayer of metal atoms is deposited onto a support, the atoms interact with the substrate. The electronic structure of the atoms can change because the distances between the individual atoms in the layer can be different from those in the pure metal. In addition, bonding between the platinum and atoms of the support can lead to undesired effects. This can greatly disrupt the catalytic properties.
Chen and his team selected tungsten carbide as a carrier. This inexpensive material has properties very similar to those of platinum. They deposited thin films of tungsten carbide onto a tungsten substrate and added platinum atoms by vapor deposition. The chemical and electronic properties of these atomic platinum monolayers on tungsten carbide did not differ significantly from those of a block of pure platinum. The catalytic efficiency of the supported platinum monolayer is also correspondingly strong.
"Tungsten carbide is the ideal substrate for platinum," says Chen. "It is possible to use significantly smaller amounts of platinum, which reduces the cost—possibly not just for water electrolysis, but also in other platinum-catalyzed processes."
Author: Jingguang G. Chen, University of Delaware, Newark (USA),
Title: Low-Cost Hydrogen-Evolution Catalysts Based on Monolayer Platinum on Tungsten Monocarbide Substrates
Angewandte Chemie International Edition
Permalink to the article: dx.doi.org/10.1002/anie.201004718
####
For more information, please click here
Copyright © Angewandte Chemie International Edition
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Fuel Cells
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Alliances/Trade associations/Partnerships/Distributorships
![]() Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
![]() University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








