Home > Press > Wax, soap clean up obstacles to better batteries
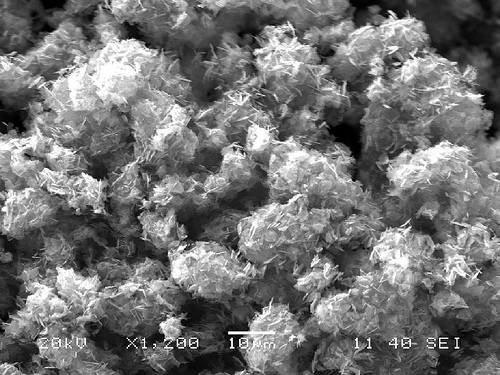 |
| Made with a one-step method, these flakes of lithium manganese phosphate can serve as electrodes for batteries. |
Abstract:
Paraffin and surfactant oleic acid improve synthesis of lithium manganese phosphate electrodes
Wax, soap clean up obstacles to better batteries
Richland, WA | Posted on August 14th, 2010A little wax and soap can help build electrodes for cheaper lithium ion batteries, according to a study in August 11 issue of Nano Letters. The one-step method will allow battery developers to explore lower-priced alternatives to the lithium ion-metal oxide batteries currently on the market.
"Paraffin provides a medium in which to grow good electrode materials," said materials scientist Daiwon Choi of the Department of Energy's Pacific Northwest National Laboratory. "This method will help researchers investigate cathode materials based on cheaper transition metals such as manganese or iron."
Consumers use long-lasting rechargeable lithium ion batteries in everything from cell phones to the latest portable gadget. Some carmakers want to use them in vehicles. Most lithium ion batteries available today are designed with an oxide of metal such as cobalt, nickel, or manganese. Choi and colleagues at PNNL and State University of New York at Binghamton wanted to explore both cheaper metals and the more stable phosphate in place of oxide.
The Recharge Tale
These rechargeable batteries work because lithium is selfish and wants its own electron. Positively charged lithium ions normally hang out in metal oxide, the stable, positive electrode in batteries. Metal oxide generously shares its electrons with the lithium ions.
Charging with electricity pumps electrons into the negative electrode, and when the lithium ions see the free-floating negative charges across the battery, they become attracted to life away from the metal oxide cage. So off the lithium ions go, abandoning the metal oxide and its shared electrons to spend time enjoying their own private ones.
But the affair doesn't last — using the battery in an electronic device creates a conduit through which the slippery electrons can flow. Losing their electrons, the lithium ions slink back to the ever-waiting metal oxide. Recharging starts the whole sordid process over.
Cheaper, Stabler
While cobalt oxide performs well in lithium batteries, cobalt and nickel are more expensive than manganese or iron. In addition, substituting phosphate for oxide provides a more stable structure for lithium.
Lithium iron phosphate batteries are commercially available in some power tools and solar products, but synthesis of the electrode material is complicated. Choi and colleagues wanted to develop a simple method to turn lithium metal phosphate into a good electrode.
Lithium manganese phosphate — LMP — can theoretically store some of the highest amounts of energy of the rechargeable batteries, weighing in at 171 milliAmp hours per gram of material. High storage capacity allows the batteries to be light. But other investigators working with LMP have not even been able to eek out 120 milliAmp hours per gram so far from the material they've synthesized.
Choi reasoned the 30 percent loss in capacity could be due to lithium and electrons having to battle their way through the metal oxide, a property called resistance. The less distance lithium and electrons have to travel out of the cathode, he thought, the less resistance and the more electricity could be stored. A smaller particle would decrease that distance.
But growing smaller particles requires lower temperatures. Unfortunately, lower temperatures means the metal oxide molecules fail to line up well in the crystals. Randomness is unsuitable for cathode materials, so the researchers needed a framework in which the ingredients — lithium, manganese and phosphate — could arrange themselves into neat crystals.
Wax On, Wax Off
Paraffin wax is made up of long straight molecules that don't react with much, and the long molecules might help line things up. Soap — a surfactant called oleic acid — might help the growing crystals disperse evenly.
So, Choi and colleagues mixed the electrode ingredients with melted paraffin and oleic acid and let the crystals grow as they slowly raised the temperature. By 400 Celsius (four times the temperature of boiling water), crystals had formed and the wax and soap had boiled off. Materials scientists generally strengthen metals by subjecting them to high heat, so the team raised the temperature even more to meld the crystals into a plate.
"This method is a lot simpler than other ways of making lithium manganese phosphate cathodes," said Choi. "Other groups have a complicated, multi-step process. We mix all the components and heat it up."
To measure the size of the miniscule plates, the team used a transmission electron microscope in EMSL, DOE's Environmental Molecular Sciences Laboratory on the PNNL campus. Up close, tiny, thin rectangles poked every which way. The nanoplates measured about 50 nanometers thick -- about a thousand times thinner than a human hair -- and up to 2000 nanometers on a side. Other analyses showed the crystal growth was suitable for electrodes.
To test LMP, the team shook the nanoplates free from one another and added a conductive carbon backing, which serves as the positive electrode. The team tested how much electricity the material could store after charging and discharging fast or slowly.
When the researchers charged the nanoplates slowly over a day and then discharged them just as slowly, the LMP mini battery held a little more than 150 milliAmp hours per gram of material, higher than other researchers had been able to attain. But when the battery was discharged fast -- say, within an hour, that dropped to about 117, comparable to other material.
Its best performance knocked at the theoretical maximum at 168 milliAmp hours per gram, when it was slowly charged and discharged over two days. Charging and discharging in an hour — a reasonable goal for use in consumer electronics — allowed it to store a measly 54 milliAmp hours per gram.
Although this version of an LMP battery charges slower than other cathode materials, Choi said the real advantage to this work is that the easy, one-step method will let them explore a wide variety of cheap materials that have traditionally been difficult to work with in developing lithium ion rechargeable batteries.
In the future, the team will change how they incorporate the carbon coating on the LMP nanoplates, which might improve their charge and discharge rates.
Reference: Daiwon Choi, Donghai Wang, In-Tae Bae, Jie Xiao, Zimin Nie, Wei Wang, Vilayanur V. Viswanathan, Yun Jung Lee, Ji-Guang Zhang, Gordon L. Graff, Zhenguo Yang, and Jun Liu, LiMnPO4 nanoplate grown via solid-state reaction in molten hydrocarbon for li-ion battery cathode, Nano Letters, DOI 10.1021/nl1007085 (pubs.acs.org/doi/abs/10.1021/nl1007085).
This work was supported by PNNL and DOE's Offices of Energy Efficiency and Renewable Energy and of Electricity Delivery and Energy Reliability.
####
About Pacific Northwest National Laboratory
Pacific Northwest National Laboratory is a Department of Energy Office of Science national laboratory where interdisciplinary teams advance science and technology and deliver solutions to America's most intractable problems in energy, the environment and national security. PNNL employs 4,700 staff, has an annual budget of nearly $1.1 billion, and has been managed by Ohio-based Battelle since the lab's inception in 1965.
EMSL, the Environmental Molecular Sciences Laboratory, is a national scientific user facility sponsored by the Department of Energy's Office of Science, Biological and Environmental Research program that is located at Pacific Northwest National Laboratory. EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. EMSL's technical experts and suite of custom and advanced instruments are unmatched. Its integrated computational and experimental capabilities enable researchers to realize fundamental scientific insights and create new technologies.
For more information, please click here
Contacts:
Mary Beckman
PNNL
(509) 375-3688
Copyright © Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
Simulating magnetization in a Heisenberg quantum spin chain April 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Possible Futures
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
With VECSELs towards the quantum internet Fraunhofer: IAF achieves record output power with VECSEL for quantum frequency converters April 5th, 2024
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Announcements
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Energy
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
![]() Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
Shedding light on unique conduction mechanisms in a new type of perovskite oxide November 17th, 2023
![]() Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
Inverted perovskite solar cell breaks 25% efficiency record: Researchers improve cell efficiency using a combination of molecules to address different November 17th, 2023
![]() The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
The efficient perovskite cells with a structured anti-reflective layer – another step towards commercialization on a wider scale October 6th, 2023
Automotive/Transportation
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Tests find no free-standing nanotubes released from tire tread wear September 8th, 2023
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
What heat can tell us about battery chemistry: using the Peltier effect to study lithium-ion cells March 8th, 2024
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Research partnerships
![]() Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
Discovery points path to flash-like memory for storing qubits: Rice find could hasten development of nonvolatile quantum memory April 5th, 2024
![]() Researchers’ approach may protect quantum computers from attacks March 8th, 2024
Researchers’ approach may protect quantum computers from attacks March 8th, 2024
![]() 'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
'Sudden death' of quantum fluctuations defies current theories of superconductivity: Study challenges the conventional wisdom of superconducting quantum transitions January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








